Revealing Chronic Granulomatous Disease in a Patient With Williams-Beuren Syndrome Using Whole Exome Sequencing
- PMID: 34804071
- PMCID: PMC8599285
- DOI: 10.3389/fimmu.2021.778133
Revealing Chronic Granulomatous Disease in a Patient With Williams-Beuren Syndrome Using Whole Exome Sequencing
Abstract
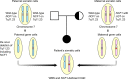
Blended phenotypes exhibited by a patient may present a challenge to the establishment of diagnosis. In this study, we report a seven-year-old Murut girl with unusual features of Williams-Beuren syndrome (WBS), including recurrent infections and skin abscesses. Considering the possibility of a second genetic disorder, a mutation screening for genes associated with inborn errors of immunity (IEI) was conducted using whole exome sequencing (WES). Analysis of copy number variations (CNVs) from the exome data revealed a 1.53Mb heterozygous deletion on chromosome 7q11.23, corresponding to the known WBS. We also identified a biallelic loss of NCF1, which indicated autosomal recessive chronic granulomatous disease (CGD). Dihydrorhodamine (DHR) flow cytometric assay demonstrated abnormally low neutrophil oxidative burst activity. Coamplification of NCF1 and its pseudogenes identified a GT-deletion (ΔGT) at the start of exon 2 in NCF1 (NM_000265.7: c.75_76delGT: p.Tyr26Hisfs*26). Estimation of NCF1-to-NCF1 pseudogenes ratio using ΔGT and 20-bp gene scans affirmed nil copies of NCF1 in the patient. While the father had a normal ratio of 2:4, the mother had a ratio of 1:5, implicating the carrier of ΔGT-containing NCF1. Discovery of a 7q11.23 deletion involving one NCF1 allele and a ΔGT in the second NCF1 allele explained the coexistence of WBS and CGD in our patient. This study highlights the capability of WES to establish a molecular diagnosis for a case with blended phenotypes, enabling the provision of appropriate prophylactic treatment.
Keywords: Williams-Beuren syndrome (WBS); blended phenotypes; chronic granulomatous disease (CGD); copy number variation (CNV); dual molecular diagnosis; whole exome sequencing (WES).
Copyright © 2021 Ripen, Chiow, Rama Rao and Mohamad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
NCF1 (p47phox)-deficient chronic granulomatous disease: comprehensive genetic and flow cytometric analysis.Blood Adv. 2019 Jan 22;3(2):136-147. doi: 10.1182/bloodadvances.2018023184. Blood Adv. 2019. PMID: 30651282 Free PMC article.
-
Functional and genetic characterization of two extremely rare cases of Williams-Beuren syndrome associated with chronic granulomatous disease.Eur J Hum Genet. 2013 Oct;21(10):1079-84. doi: 10.1038/ejhg.2012.310. Epub 2013 Jan 23. Eur J Hum Genet. 2013. PMID: 23340515 Free PMC article.
-
Approach to Molecular Diagnosis of Chronic Granulomatous Disease (CGD): an Experience from a Large Cohort of 90 Indian Patients.J Clin Immunol. 2018 Nov;38(8):898-916. doi: 10.1007/s10875-018-0567-y. Epub 2018 Nov 23. J Clin Immunol. 2018. PMID: 30470980
-
Chronic granulomatous disease: a 25-year patient registry based on a multistep diagnostic procedure, from the referral center for primary immunodeficiencies in Greece.J Clin Immunol. 2013 Nov;33(8):1302-9. doi: 10.1007/s10875-013-9940-z. Epub 2013 Oct 1. J Clin Immunol. 2013. PMID: 24081483 Review.
-
Copy number variants at Williams-Beuren syndrome 7q11.23 region.Hum Genet. 2010 Jul;128(1):3-26. doi: 10.1007/s00439-010-0827-2. Epub 2010 May 1. Hum Genet. 2010. PMID: 20437059 Review.
Cited by
-
Visual inspection reveals a novel pathogenic mutation in PKD1 missed by the variant caller in whole‑exome sequencing.Mol Med Rep. 2022 Dec;26(6):365. doi: 10.3892/mmr.2022.12882. Epub 2022 Oct 25. Mol Med Rep. 2022. PMID: 36281931 Free PMC article.
-
Case Report: ISG15 deficiency caused by novel variants in two families and effective treatment with Janus kinase inhibition.Front Immunol. 2023 Dec 5;14:1287258. doi: 10.3389/fimmu.2023.1287258. eCollection 2023. Front Immunol. 2023. PMID: 38115997 Free PMC article.
-
2q33 Deletions Underlying Syndromic and Non-syndromic CTLA4 Deficiency.J Clin Immunol. 2024 Nov 23;45(1):46. doi: 10.1007/s10875-024-01831-5. J Clin Immunol. 2024. PMID: 39578275
References
-
- Davies JH, Moon RJ. Hypercalcemia. In: Huhtaniemi I, Martini L, editors. Encyclopedia of Endocrine Diseases, 2nd ed. Oxford: Academic Press; (2019). p. 366–77.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

