Metabolic Imbalance Effect on Retinal Müller Glial Cells Reprogramming Capacity: Involvement of Histone Deacetylase SIRT6
- PMID: 34804128
- PMCID: PMC8599966
- DOI: 10.3389/fgene.2021.769723
Metabolic Imbalance Effect on Retinal Müller Glial Cells Reprogramming Capacity: Involvement of Histone Deacetylase SIRT6
Abstract
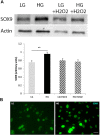
Retinal Müller glial cells (MGs) are among the first to demonstrate metabolic changes during retinal disease and are a potential source of regenerative cells. In response to a harmful stimulus, they can dedifferentiate acquiring neural stem cells properties, proliferate and migrate to the damaged retinal layer and differentiate into lost neurons. However, it is not yet known how this reprogramming process is regulated in mammals. Since glucose and oxygen are important regulatory elements that may help directing stem cell fate, we aimed to study the effect of glucose variations and oxidative stress in Müller cells reprogramming capacity and analyze the participation the histone deacetylase SIRT6, as an epigenetic modulator of this process. We found that the combination of high glucose and oxidative stress induced a decrease in the levels of the marker glutamine synthetase, and an increase in the migration capacity of the cells suggesting that these experimental conditions could induce some degree of dedifferentiation and favor the migration ability. High glucose induced an increase in the levels of the pluripotent factor SOX9 and a decrease in SIRT6 levels accompanied by the increase in the acetylation levels of H3K9. Inhibiting SIRT6 expression by siRNA rendered an increase in SOX9 levels. We also determined SOX9 levels in retinas from mice with a conditional deletion of SIRT6 in the CNS. To further understand the mechanisms that regulate MGs response under metabolic impaired conditions, we evaluated the gene expression profile and performed Gene Ontology enrichment analysis of Müller cells from a murine model of Diabetes. We found several differentially expressed genes and observed that the transcriptomic change involved the enrichment of genes associated with glucose metabolism, cell migration, development and pluripotency. We found that many functional categories affected in cells of diabetic animals were directly related to SIRT6 function. Transcription factors enrichment analysis allowed us to predict several factors, including SOX9, that may be involved in the modulation of the differential expression program observed in diabetic MGs. Our results underline the heterogeneity of Müller cells response and the challenge that the study of metabolic impairment in vivo represents.
Keywords: SIRT6; metabolism; müller cells; reprogramming; retina.
Copyright © 2021 Sanhueza Salas, García-Venzor, Beltramone, Capurro, Toiber and Silberman.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials

