Synthetic strategies toward 1,3-oxathiolane nucleoside analogues
- PMID: 34804240
- PMCID: PMC8576827
- DOI: 10.3762/bjoc.17.182
Synthetic strategies toward 1,3-oxathiolane nucleoside analogues
Abstract
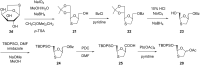
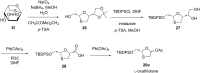
Sugar-modified nucleosides have gained considerable attention in the scientific community, either for use as molecular probes or as therapeutic agents. When the methylene group of the ribose ring is replaced with a sulfur atom at the 3'-position, these compounds have proved to be structurally potent nucleoside analogues, and the best example is BCH-189. The majority of methods traditionally involves the chemical modification of nucleoside structures. It requires the creation of artificial sugars, which is accompanied by coupling nucleobases via N-glycosylation. However, over the last three decades, efforts were made for the synthesis of 1,3-oxathiolane nucleosides by selective N-glycosylation of carbohydrate precursors at C-1, and this approach has emerged as a strong alternative that allows simple modification. This review aims to provide a comprehensive overview on the reported methods in the literature to access 1,3-oxathiolane nucleosides. The first focus of this review is the construction of the 1,3-oxathiolane ring from different starting materials. The second focus involves the coupling of the 1,3-oxathiolane ring with different nucleobases in a way that only one isomer is produced in a stereoselective manner via N-glycosylation. An emphasis has been placed on the C-N-glycosidic bond constructed during the formation of the nucleoside analogue. The third focus is on the separation of enantiomers of 1,3-oxathiolane nucleosides via resolution methods. The chemical as well as enzymatic procedures are reviewed and segregated in this review for effective synthesis of 1,3-oxathiolane nucleoside analogues.
Keywords: 1,3-oxathiolane sugar and nucleosides; Lewis acids; N-glycosylation; chiral auxiliaries; enzymes; separation of racemic nucleosides; stereoselectivity.
Copyright © 2021, Aher et al.
Figures
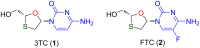


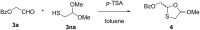



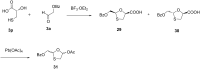

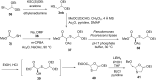
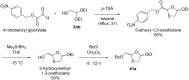

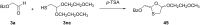
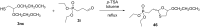
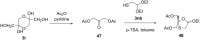
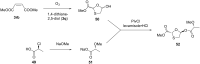


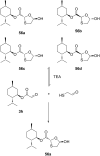

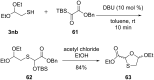
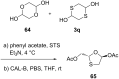



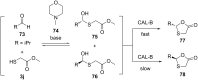
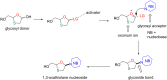


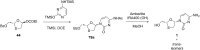




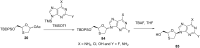
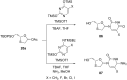

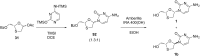

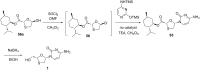




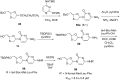


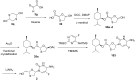
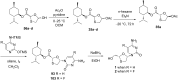
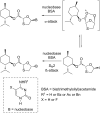

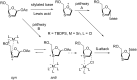

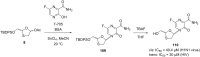

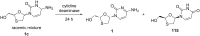
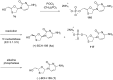

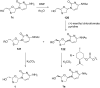

References
-
- Wilson L J, Hager M W, El-Kattan Y A, Liotta D C. Synthesis. 1995;(12):1465–1479. doi: 10.1055/s-1995-4142. - DOI
-
- [Jun 21;2021 ];Cancer. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer.
Publication types
LinkOut - more resources
Full Text Sources
