Targeting the Ang2/Tie2 Axis with Tanshinone IIA Elicits Vascular Normalization in Ischemic Injury and Colon Cancer
- PMID: 34804370
- PMCID: PMC8598375
- DOI: 10.1155/2021/7037786
Targeting the Ang2/Tie2 Axis with Tanshinone IIA Elicits Vascular Normalization in Ischemic Injury and Colon Cancer
Abstract
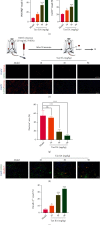
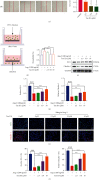
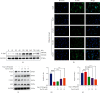
Pathological angiogenesis, as exhibited by aberrant vascular structure and function, has been well deemed to be a hallmark of cancer and various ischemic diseases. Therefore, strategies to normalize vasculature are of potential therapeutic interest in these diseases. Recently, identifying bioactive compounds from medicinal plant extracts to reverse abnormal vasculature has been gaining increasing attention. Tanshinone IIA (Tan IIA), an active component of Salvia miltiorrhiza, has been shown to play significant roles in improving blood circulation and delaying tumor progression. However, the underlying mechanisms responsible for the therapeutic effects of Tan IIA are not fully understood. Herein, we established animal models of HT-29 human colon cancer xenograft and hind limb ischemia to investigate the role of Tan IIA in regulating abnormal vasculature. Interestingly, our results demonstrated that Tan IIA could significantly promote the blood flow, alleviate the hypoxia, improve the muscle quality, and ameliorate the pathological damage after ischemic insult. Meanwhile, we also revealed that Tan IIA promoted the integrity of vascular structure, reduced vascular leakage, and attenuated the hypoxia in HT-29 tumors. Moreover, the circulating angiopoietin 2 (Ang2), which is extremely high in these two pathological states, was substantially depleted in the presence of Tan IIA. Also, the activation of Tie2 was potentiated by Tan IIA, resulting in decreased vascular permeability and elevated vascular integrity. Mechanistically, we uncovered that Tan IIA maintained vascular stability by targeting the Ang2-Tie2-AKT-MLCK cascade. Collectively, our data suggest that Tan IIA normalizes vessels in tumors and ischemic injury via regulating the Ang2/Tie2 signaling pathway.
Copyright © 2021 Wei Zou et al.
Conflict of interest statement
The authors declared no conflict of interest.
Figures



Similar articles
-
Integrated identification and mechanism exploration of bioactive ingredients from Salvia miltiorrhiza to induce vascular normalization.Phytomedicine. 2025 Mar;138:156427. doi: 10.1016/j.phymed.2025.156427. Epub 2025 Jan 25. Phytomedicine. 2025. PMID: 39892310
-
Tanshinone IIA induces ER stress and JNK activation to inhibit tumor growth and enhance anti-PD-1 immunotherapy in non-small cell lung cancer.Phytomedicine. 2024 Jun;128:155431. doi: 10.1016/j.phymed.2024.155431. Epub 2024 Feb 7. Phytomedicine. 2024. PMID: 38537440
-
Tanshinone IIA induces ferroptosis in gastric cancer cells through p53-mediated SLC7A11 down-regulation.Biosci Rep. 2020 Aug 28;40(8):BSR20201807. doi: 10.1042/BSR20201807. Biosci Rep. 2020. PMID: 32776119 Free PMC article.
-
Pharmacological Activity and Mechanism of Tanshinone IIA in Related Diseases.Drug Des Devel Ther. 2020 Nov 5;14:4735-4748. doi: 10.2147/DDDT.S266911. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 33192051 Free PMC article. Review.
-
Tanshinone IIA targeting cell signaling pathways: a plausible paradigm for cancer therapy.Pharmacol Rep. 2023 Aug;75(4):907-922. doi: 10.1007/s43440-023-00507-y. Epub 2023 Jul 13. Pharmacol Rep. 2023. PMID: 37440106 Review.
Cited by
-
Complications and comorbidities associated with antineoplastic chemotherapy: Rethinking drug design and delivery for anticancer therapy.Acta Pharm Sin B. 2024 Jul;14(7):2901-2926. doi: 10.1016/j.apsb.2024.03.006. Epub 2024 Mar 11. Acta Pharm Sin B. 2024. PMID: 39027258 Free PMC article. Review.
-
Transgelin-2 Involves in the Apoptosis of Colorectal Cancer Cells Induced by Tanshinone-IIA.Anal Cell Pathol (Amst). 2022 Sep 27;2022:9358583. doi: 10.1155/2022/9358583. eCollection 2022. Anal Cell Pathol (Amst). 2022. PMID: 36204303 Free PMC article.
-
The mechanisms of tanshinone in the treatment of tumors.Front Pharmacol. 2023 Oct 26;14:1282203. doi: 10.3389/fphar.2023.1282203. eCollection 2023. Front Pharmacol. 2023. PMID: 37964867 Free PMC article. Review.
-
Sodium tanshinone IIA sulphate inhibits angiogenesis in lung adenocarcinoma via mediation of miR-874/eEF-2K/TG2 axis.Pharm Biol. 2023 Dec;61(1):868-877. doi: 10.1080/13880209.2023.2204879. Pharm Biol. 2023. PMID: 37300283 Free PMC article.
-
Tanshinone ΙΙA-Incubated Mesenchymal Stem Cells Inhibit Lipopolysaccharide-Induced Inflammation of N9 Cells through TREM2 Signaling Pathway.Stem Cells Int. 2022 Mar 4;2022:9977610. doi: 10.1155/2022/9977610. eCollection 2022. Stem Cells Int. 2022. PMID: 35283996 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

