The environmental adaptation strategy of seed germination, and roles of the seed pappus on dispersal and hypocotyl hairs on seedling anchorage in Tamarix ramosissima
- PMID: 34804468
- PMCID: PMC8600553
- DOI: 10.1093/aobpla/plab065
The environmental adaptation strategy of seed germination, and roles of the seed pappus on dispersal and hypocotyl hairs on seedling anchorage in Tamarix ramosissima
Abstract
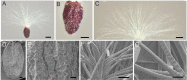
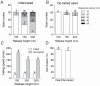
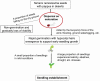
Seed dispersal, germination and seedling establishment are affected by various ecological factors in desert plant species. Tamarix ramosissima has evolved multiple strategies to facilitate its survival in harsh environments during the early stages of development. In this study, we investigated the effects of different ecological factors on seed germination and seedling growth, the function of the seed pappus in seed dispersal, as well as the function of the hypocotyl hairs in seedling establishment. We found that the seed germination of T. ramosissima was rapid and could occur under a wide range of temperatures (5-30 °C), after long periods of storage (at least 12 months on dispersal), under high concentrations of salts (700-900 mmol·L-1) and polyethylene glycol (PEG) 6000 (500 g·L-1) and under medium concentrations of alkalis (300-500 mmol·L-1). Lower concentrations of salts and PEG promoted seedling growth. The seed pappus had no effect on seed germination, but it might function as an accessory structure that provides a buoyancy force and promotes long-distance seed dispersal. The hypocotyl hairs located on the edge of the hypocotyl end might aid the upright positioning of the seedlings during early development, especially when seed germination occurs under floating or flooding conditions. In conclusion, the germination of T. ramosissima seeds and seedling development can occur under diverse types of abiotic stress, and the seed pappus and hypocotyl hairs played an important role in seed dispersal and seedling establishment.
Keywords: Hypocotyl hairs; Tamarix ramosissima; seed dispersal; seed germination; seed pappus.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Annals of Botany Company.
Figures





Similar articles
-
Comparative germination of Tamarix ramosissima spring and summer seeds.EXCLI J. 2011 Oct 14;10:198-204. eCollection 2011. EXCLI J. 2011. PMID: 27857674 Free PMC article.
-
The effect of seed-dispersal timing on seedling recruitment is modulated by environmental conditions that vary across altitude in a threatened palm.Ann Bot. 2022 Jul 18;129(7):839-856. doi: 10.1093/aob/mcac038. Ann Bot. 2022. PMID: 35325032 Free PMC article.
-
Seed germination and early seedling survival of the invasive species Prosopis juliflora (Fabaceae) depend on habitat and seed dispersal mode in the Caatinga dry forest.PeerJ. 2020 Sep 3;8:e9607. doi: 10.7717/peerj.9607. eCollection 2020. PeerJ. 2020. PMID: 32953255 Free PMC article.
-
[Adaptation strategies of seed germination and seedling growth to sand dune environment].Ying Yong Sheng Tai Xue Bao. 2006 Jan;17(1):137-42. Ying Yong Sheng Tai Xue Bao. 2006. PMID: 16689250 Review. Chinese.
-
The Dead Can Nurture: Novel Insights into the Function of Dead Organs Enclosing Embryos.Int J Mol Sci. 2018 Aug 19;19(8):2455. doi: 10.3390/ijms19082455. Int J Mol Sci. 2018. PMID: 30126259 Free PMC article. Review.
Cited by
-
Structural modifications in Bermuda grass [Cynodon dactylon (L.) Pers.] ecotypes for adaptation to environmental heterogeneity.Front Plant Sci. 2023 Jan 19;13:1084706. doi: 10.3389/fpls.2022.1084706. eCollection 2022. Front Plant Sci. 2023. PMID: 36756232 Free PMC article.
-
The Developmental Delay of Seedlings With Cotyledons Only Confers Stress Tolerance to Suaeda aralocaspica (Chenopodiaceae) by Unique Performance on Morphology, Physiology, and Gene Expression.Front Plant Sci. 2022 Jun 6;13:844430. doi: 10.3389/fpls.2022.844430. eCollection 2022. Front Plant Sci. 2022. PMID: 35734249 Free PMC article.
References
-
- Aleman R, Jusaitis M, Gibbs J, Ainsley P, Tiver F, Petit S. 2015. Influence of seed dimorphism and provenance on seed morphology, dispersal, germination and seedling growth of Brachyscome ciliaris (Asteraceae). Australian Journal of Botany 63:705–713.
-
- Al-Hawija BN, Partzsch M, Hensen I. 2012. Effects of temperature, salinity and cold stratification on seed germination in halophytes. Nordic Journal of Botany 30:627–634.
-
- Andersen MC. 1993. Diaspore morphology and seed dispersal in several wind-dispersed Asteraceae. American Journal of Botany 80:487–492. - PubMed
-
- Aronne G, Micco VD. 2004. Hypocotyl features of Myrtus communis (Myrtaceae): a many-sided strategy for possible enhancement of seedling establishment in the Mediterranean environment. Botanical Journal of the Linnean Society 145:195–202.
-
- Benvenuti S, Macchia M, Miele S. 2001. Light, temperature and burial depth effects on Rumex obtusifolius seed germination and emergence. Weed Research 41:177–186.
LinkOut - more resources
Full Text Sources

