Mitochondrial Mechanisms of Apoptosis and Necroptosis in Liver Diseases
- PMID: 34804779
- PMCID: PMC8601834
- DOI: 10.1155/2021/8900122
Mitochondrial Mechanisms of Apoptosis and Necroptosis in Liver Diseases
Abstract
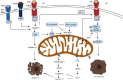
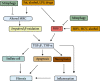
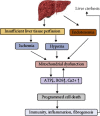
In addition to playing a pivotal role in cellular energetics and biosynthesis, mitochondrial components are key operators in the regulation of cell death. In addition to apoptosis, necrosis is a highly relevant form of programmed liver cell death. Differential activation of specific forms of programmed cell death may not only affect the outcome of liver disease but may also provide new opportunities for therapeutic intervention. This review describes the role of mitochondria in cell death and the mechanism that leads to chronic liver hepatitis and liver cirrhosis. We focus on mitochondrial-driven apoptosis and current knowledge of necroptosis and discuss therapeutic strategies for targeting mitochondrial-mediated cell death in liver diseases.
Copyright © 2021 Qingfei Chu et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
Apoptosis and necroptosis in the liver: a matter of life and death.Nat Rev Gastroenterol Hepatol. 2018 Dec;15(12):738-752. doi: 10.1038/s41575-018-0065-y. Nat Rev Gastroenterol Hepatol. 2018. PMID: 30250076 Free PMC article. Review.
-
Lytic cell death in metabolic liver disease.J Hepatol. 2020 Aug;73(2):394-408. doi: 10.1016/j.jhep.2020.04.001. Epub 2020 Apr 13. J Hepatol. 2020. PMID: 32298766 Free PMC article. Review.
-
Mitochondrial Mechanisms of Necroptosis in Liver Diseases.Int J Mol Sci. 2020 Dec 23;22(1):66. doi: 10.3390/ijms22010066. Int J Mol Sci. 2020. PMID: 33374660 Free PMC article. Review.
-
Necroptosis: an emerging type of cell death in liver diseases.World J Gastroenterol. 2014 Sep 21;20(35):12526-32. doi: 10.3748/wjg.v20.i35.12526. World J Gastroenterol. 2014. PMID: 25253954 Free PMC article. Review.
-
Molecular Insights into the Mechanism of Necroptosis: The Necrosome As a Potential Therapeutic Target.Cells. 2019 Nov 21;8(12):1486. doi: 10.3390/cells8121486. Cells. 2019. PMID: 31766571 Free PMC article. Review.
Cited by
-
Protective effect of traditional Chinese medicine on non-alcoholic fatty liver disease and liver cancer by targeting ferroptosis.Front Nutr. 2022 Oct 18;9:1033129. doi: 10.3389/fnut.2022.1033129. eCollection 2022. Front Nutr. 2022. PMID: 36330148 Free PMC article. Review.
-
Patients with hepatocellular carcinoma that die during the first year of liver transplantation have high blood sFasL concentrations.World J Clin Cases. 2023 Mar 16;11(8):1753-1760. doi: 10.12998/wjcc.v11.i8.1753. World J Clin Cases. 2023. PMID: 36970008 Free PMC article.
-
Imbalance of mitochondrial fusion in peripheral blood mononuclear cells is associated with liver fibrosis in patients with metabolic dysfunction-associated steatohepatitis.Heliyon. 2024 Mar 8;10(6):e27557. doi: 10.1016/j.heliyon.2024.e27557. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38496899 Free PMC article.
-
Effect of doramectin on programmed cell death pathway in glioma cells.Clin Transl Oncol. 2023 Oct;25(10):2871-2883. doi: 10.1007/s12094-023-03147-z. Epub 2023 Apr 21. Clin Transl Oncol. 2023. PMID: 37084153
-
Investigating the Neuroprotective and Cognitive-Enhancing Effects of Bacopa monnieri: A Systematic Review Focused on Inflammation, Oxidative Stress, Mitochondrial Dysfunction, and Apoptosis.Antioxidants (Basel). 2024 Mar 25;13(4):393. doi: 10.3390/antiox13040393. Antioxidants (Basel). 2024. PMID: 38671841 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

