Adoption of adjuvant bisphosphonates for early breast cancer into standard clinical practice: Challenges and lessons learnt from comparison of the UK and Australian experience
- PMID: 34804788
- PMCID: PMC8581365
- DOI: 10.1016/j.jbo.2021.100402
Adoption of adjuvant bisphosphonates for early breast cancer into standard clinical practice: Challenges and lessons learnt from comparison of the UK and Australian experience
Abstract
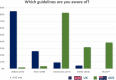
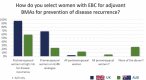
International guidelines recommend adjuvant bisphosphonates (BPs) for post-menopausal women with early breast cancer to reduce recurrence and mortality. However, globally, wide variation exists in their adoption. In the UK, adjuvant BPs were a recommendation in the breast cancer Clinical Reference Group service specification and were included as a priority for implementation by the national oncologists group UK Breast Cancer Group in November 2015, promoting national uptake, guidance and funding arrangements. In 2018, adjuvant BPs were recommended by the UKs National Institute for Health and Care Excellence. In Australia, adjuvant BPs are still 'off-label' and do not receive national reimbursement or endorsement. To date there has been no research into the prescribing habits of these agents in Australia. With the aim to gather data on adjuvant BPs prescribing practices, online surveys were developed and disseminated to breast oncologists in both countries between December 2018 and June 2019. Almost all of the UK oncologists prescribed adjuvant BPs, demonstrating that education, endorsement from professional bodies, presence of national guidelines and funding decisions have been critical to implementation. In contrast, only 48% of the Australian responders prescribed adjuvant BPs, while 83% reported that they would prescribe them if funding was available. Lack of local protocol guidance was also seen as a major barrier. This study was intended to assess the pathway taken for adjuvant BP implementation in the UK and how it might inform changes in Australian practice and also guide other countries with similar issues with the ultimate aim of improving the care of women with early breast cancer globally.
Keywords: Adjuvant; Bisphosphonates; Breast cancer; Post-menopausal; Survival.
© 2021 The Authors. Published by Elsevier GmbH.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Compliance and patient reported toxicity from oral adjuvant bisphosphonates in patients with early breast cancer. A cross sectional study.J Bone Oncol. 2019 Feb 19;15:100226. doi: 10.1016/j.jbo.2019.100226. eCollection 2019 Apr. J Bone Oncol. 2019. PMID: 30937280 Free PMC article.
-
Beyond the black stump: rapid reviews of health research issues affecting regional, rural and remote Australia.Med J Aust. 2020 Dec;213 Suppl 11:S3-S32.e1. doi: 10.5694/mja2.50881. Med J Aust. 2020. PMID: 33314144
-
Bisphosphonates in the adjuvant treatment of young women with breast cancer: the estrogen rich is a poor candidate!J Thorac Dis. 2013 Jun;5 Suppl 1(Suppl 1):S27-35. doi: 10.3978/j.issn.2072-1439.2013.06.04. J Thorac Dis. 2013. PMID: 23819025 Free PMC article.
-
Bisphosphonates for cancer patients: why, how, and when?Support Care Cancer. 2002 Jul;10(5):399-407. doi: 10.1007/s005200100292. Epub 2001 Oct 19. Support Care Cancer. 2002. PMID: 12136223 Review.
-
National Institutes of Health Consensus Development Conference statement: adjuvant therapy for breast cancer, November 1-3, 2000.J Natl Cancer Inst Monogr. 2001;(30):5-15. J Natl Cancer Inst Monogr. 2001. PMID: 11773285 Review.
Cited by
-
Translational researchers' training and development needs, preferences, and barriers: A survey in a National Institute for Health Research Biomedical Research Centre in the United Kingdom.Clin Transl Sci. 2022 Jul;15(7):1737-1752. doi: 10.1111/cts.13289. Epub 2022 May 15. Clin Transl Sci. 2022. PMID: 35570378 Free PMC article.
-
Clinical and economic research of bone modifiers as adjuvant therapy for early breast cancer: A systematic literature review.Breast. 2025 Aug 7;83:104551. doi: 10.1016/j.breast.2025.104551. Online ahead of print. Breast. 2025. PMID: 40803152 Free PMC article. Review.
-
Benefit of adjuvant bisphosphonates in early breast cancer treated with contemporary systemic therapy: A meta-analysis of randomized control trials.Heliyon. 2024 Jan 19;10(2):e24793. doi: 10.1016/j.heliyon.2024.e24793. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38312616 Free PMC article.
-
The effect of multidisciplinary team on survival rates of women with breast cancer: a systematic review and meta-analysis.Ann Med Surg (Lond). 2023 May 25;85(6):2940-2948. doi: 10.1097/MS9.0000000000000914. eCollection 2023 Jun. Ann Med Surg (Lond). 2023. PMID: 37363480 Free PMC article.
-
Patterns of adjuvant bone modifying agent use in patients with early-stage breast cancer in the United States.Breast Cancer Res. 2025 Jun 11;27(1):102. doi: 10.1186/s13058-025-02062-1. Breast Cancer Res. 2025. PMID: 40500761 Free PMC article.
References
-
- World Health Organisation, Breast cancer. https://www.who.int/cancer/prevention/diagnosis-screening/breast-cancer/... (accessed 23 May 2019)
-
- Breast Cancer Now, Facts and statistics 2021. https://breastcancernow.org/about-us/media/facts-statistics, 2021 (accessed 25 March 2021).
-
- Australian Institute of Health and Welfare & Cancer Australia 2012. Breast cancer in Australia: an overview. Cancer series no. 71. Cat. no. CAN 67. Canberra: AIHW.
-
- Cancer Australia, Breast cancer statistics. https://breast-cancer.canceraustralia.gov.au/statistics, 2019 (accessed 29 July 2019).
-
- Powles T., Paterson A., McCloskey E., Schein P., Scheffler B., Tidy A., Ashley S., Smith I., Ottestad L., Kanis J. Correction: Reduction in bone relapse and improved survival with oral clodronate for adjuvant treatment of operable breast cancer [ISRCTN83688026] Breast Cancer Res. 2006;8(3):406. doi: 10.1186/bcr1413. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

