Novel Nanoparticle-Based Cancer Treatment, Effectively Inhibits Lung Metastases and Improves Survival in a Murine Breast Cancer Model
- PMID: 34804962
- PMCID: PMC8602876
- DOI: 10.3389/fonc.2021.761045
Novel Nanoparticle-Based Cancer Treatment, Effectively Inhibits Lung Metastases and Improves Survival in a Murine Breast Cancer Model
Abstract
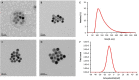
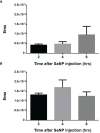
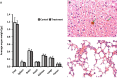
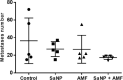
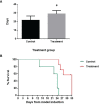
Sarah Nanoparticles (SaNPs) are unique multicore iron oxide-based nanoparticles, developed for the treatment of advanced cancer, following standard care, through the selective delivery of thermal energy to malignant cells upon exposure to an alternating magnetic field. For their therapeutic effect, SaNPs need to accumulate in the tumor. Since the potential accumulation and associated toxicity in normal tissues are an important risk consideration, biodistribution and toxicity were assessed in naïve BALB/c mice. Therapeutic efficacy and the effect on survival were investigated in the 4T1 murine model of metastatic breast cancer. Toxicity evaluation at various timepoints did not reveal any abnormal clinical signs, evidence of alterations in organ function, nor histopathologic adverse target organ toxicity, even after a follow up period of 25 weeks, confirming the safety of SaNP use. The biodistribution evaluation, following SaNP administration, indicated that SaNPs accumulate mainly in the liver and spleen. A comprehensive pharmacokinetics evaluation, demonstrated that the total percentage of SaNPs that accumulated in the blood and vital organs was ~78%, 46%, and 36% after 4, 13, and 25 weeks, respectively, suggesting a time-dependent clearance from the body. Efficacy studies in mice bearing 4T1 metastatic tumors revealed a 49.6% and 70% reduction in the number of lung metastases and their relative size, respectively, in treated vs. control mice, accompanied by a decrease in tumor cell viability in response to treatment. Moreover, SaNP treatment followed by alternating magnetic field exposure significantly improved the survival rate of treated mice compared to the controls. The median survival time was 29 ± 3.8 days in the treated group vs. 21.6 ± 4.9 days in the control, p-value 0.029. These assessments open new avenues for generating SaNPs and alternating magnetic field application as a potential novel therapeutic modality for metastatic cancer patients.
Keywords: alternating magnetic field (AMF); enhanced permeability and retention (EPR) effect; iron oxide nanoparticles; magnetic hyperthermia (MHT); metastatic breast cancer (BC).
Copyright © 2021 Kraus, Khandadash, Hof, Nyska, Sigalov, Eltanani, Rukenstein, Rabinovitz, Kassem, Antebi, Shalev, Cohen-Erner, Goss and Cyjon.
Conflict of interest statement
Authors SK, RKD, RH, ES, ME, PR, RR, RK, AA, OS, and MC-E were employed by New Phase Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from New Phase Ltd. The funder had the following involvement with the study: Study design, collection, analysis, interpretation of data, and the writing of this article.
Figures




References
-
- Rabinovitz R, Shalev O, Kraus S. Novel Approach for Magnetic Nanoparticle-Based Hyperthermia for Metastatic Cancer Treatment. BioMed J Sci Tech Res (2020) 32:25025–27. doi: 10.26717/BJSTR.2020.32.005254 - DOI
LinkOut - more resources
Full Text Sources

