Electronic Data Management for Vaccine Trials in Low Resource Settings: Upgrades, Scalability, and Impact of ODK
- PMID: 34805059
- PMCID: PMC8599145
- DOI: 10.3389/fpubh.2021.665584
Electronic Data Management for Vaccine Trials in Low Resource Settings: Upgrades, Scalability, and Impact of ODK
Abstract
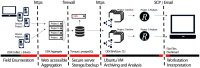
Background: ODK provides software and standards that are popular solutions for off-grid electronic data collection and has substantial code overlap and interoperability with a number of related software products including CommCare, Enketo, Ona, SurveyCTO, and KoBoToolbox. These tools provide open-source options for off-grid use in public health data collection, management, analysis, and reporting. During the 2018-2020 Ebola epidemic in the North Kivu and Ituri regions of Democratic Republic of Congo, we used these tools to support the DRC Ministère de la Santé RDC and World Health Organization in their efforts to administer an experimental vaccine (VSV-Zebov-GP) as part of their strategy to control the transmission of infection. Method: New functions were developed to facilitate the use of ODK, Enketo and R in large scale data collection, aggregation, monitoring, and near-real-time analysis during clinical research in health emergencies. We present enhancements to ODK that include a built-in audit-trail, a framework and companion app for biometric registration of ISO/IEC 19794-2 fingerprint templates, enhanced performance features, better scalability for studies featuring millions of data form submissions, increased options for parallelization of research projects, and pipelines for automated management and analysis of data. We also developed novel encryption protocols for enhanced web-form security in Enketo. Results: Against the backdrop of a complex and challenging epidemic response, our enhanced platform of open tools was used to collect and manage data from more than 280,000 eligible study participants who received VSV-Zebov-GP under informed consent. These data were used to determine whether the VSV-Zebov-GP was safe and effective and to guide daily field operations. Conclusions: We present open-source developments that make electronic data management during clinical research and health emergencies more viable and robust. These developments will also enhance and expand the functionality of a diverse range of data collection platforms that are based on the ODK software and standards.
Keywords: Ebola; electronic data collection; low and middle income countries; open source software; open-data-kit.
Copyright © 2021 Marks, Lal, Brindle, Gsell, MacGregor, Stott, van de Rijdt, Almazor, Golia, Watson, Diallo, Toure, Houlihan, Keating, Martin, Restrepo, Anokwa and Roberts.
Conflict of interest statement
CS, GA, HM, and YA work for Nafundi LLC, a company that maintains ODK. MR is the lead developer, founder and owner of Enketo LLC. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Biometric linkage of longitudinally collected electronic case report forms and confirmation of subject identity: an open framework for ODK and related tools.Front Digit Health. 2023 Aug 4;5:1072331. doi: 10.3389/fdgth.2023.1072331. eCollection 2023. Front Digit Health. 2023. PMID: 37600479 Free PMC article.
-
Protocol for a phase 3 trial to evaluate the effectiveness and safety of a heterologous, two-dose vaccine for Ebola virus disease in the Democratic Republic of the Congo.BMJ Open. 2022 Mar 8;12(3):e055596. doi: 10.1136/bmjopen-2021-055596. BMJ Open. 2022. PMID: 35260458 Free PMC article.
-
Effectiveness of rVSV-ZEBOV vaccination during the 2018-20 Ebola virus disease epidemic in the Democratic Republic of the Congo: a retrospective test-negative study.Lancet Infect Dis. 2024 Dec;24(12):1357-1365. doi: 10.1016/S1473-3099(24)00419-5. Epub 2024 Aug 20. Lancet Infect Dis. 2024. PMID: 39178866
-
Impact of Ebola epidemics on the daily operation of existing systems in Eastern Democratic Republic of the Congo: a brief review.J Med Econ. 2024 Jan-Dec;27(1):184-192. doi: 10.1080/13696998.2024.2305009. Epub 2024 Jan 22. J Med Econ. 2024. PMID: 38240249 Review.
-
Correlates of vaccine-induced protective immunity against Ebola virus disease.Semin Immunol. 2018 Oct;39:65-72. doi: 10.1016/j.smim.2018.07.003. Epub 2018 Jul 21. Semin Immunol. 2018. PMID: 30041831 Review.
Cited by
-
Predicting mortality in febrile adults: comparative performance of the MEWS, qSOFA, and UVA scores using prospectively collected data among patients in four health-care sites in sub-Saharan Africa and South-Eastern Asia.EClinicalMedicine. 2024 Oct 4;77:102856. doi: 10.1016/j.eclinm.2024.102856. eCollection 2024 Nov. EClinicalMedicine. 2024. PMID: 39416389 Free PMC article.
-
Developing and assessing the efficiency of VOSER software in recording dental caries according to WHO's criteria 2013.J Dent Sci. 2023 Oct;18(4):1822-1829. doi: 10.1016/j.jds.2023.06.019. Epub 2023 Jul 18. J Dent Sci. 2023. PMID: 37799859 Free PMC article.
-
Using an analogue-digital hybrid clinical data management platform during a two-dose preventive Ebola virus vaccine trial in Goma, the Democratic Republic of the Congo.PLOS Glob Public Health. 2025 May 2;5(5):e0004487. doi: 10.1371/journal.pgph.0004487. eCollection 2025. PLOS Glob Public Health. 2025. PMID: 40315243 Free PMC article.
-
Vaccine Confidence and Hesitancy at the Start of COVID-19 Vaccine Deployment in the UK: An Embedded Mixed-Methods Study.Front Public Health. 2021 Nov 11;9:745630. doi: 10.3389/fpubh.2021.745630. eCollection 2021. Front Public Health. 2021. PMID: 34858927 Free PMC article.
-
Spatiotemporal dynamics of the oropharyngeal microbiome in a cohort of Ivorian school children.Sci Rep. 2024 Dec 28;14(1):30895. doi: 10.1038/s41598-024-81829-6. Sci Rep. 2024. PMID: 39730689 Free PMC article.
References
-
- ODK . Available online at: https://Getodk.org/ (Accessed June 16, 2020).
-
- Phiri TB, Kaunda-Khangamwa BN, Bauleni A, Chimuna T, Melody D, Kalengamaliro H, et al. . Feasibility, acceptability and impact of integrating malaria rapid diagnostic tests and pre-referral rectal artesunate into the integrated community case management programme. A pilot study in Mchinji district, Malawi. Malar J. (2016) 15:177. 10.1186/s12936-016-1237-2 - DOI - PMC - PubMed
-
- Fornace KM, Surendra H, Abidin TR, Reyes R, Macalinao MLM, Stresman G, et al. . Use of mobile technology-based participatory mapping approaches to geolocate health facility attendees for disease surveillance in low resource settings. Int J Health Geogr. (2018) 17:21. 10.1186/s12942-018-0141-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

