Robust Agrobacterium-Mediated Transient Expression in Two Duckweed Species (Lemnaceae) Directed by Non-replicating, Replicating, and Cell-to-Cell Spreading Vectors
- PMID: 34805101
- PMCID: PMC8600122
- DOI: 10.3389/fbioe.2021.761073
Robust Agrobacterium-Mediated Transient Expression in Two Duckweed Species (Lemnaceae) Directed by Non-replicating, Replicating, and Cell-to-Cell Spreading Vectors
Abstract
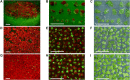
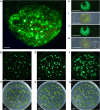
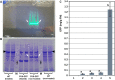
Plant-based transient expression systems have recognized potential for use as rapid and cost-effective alternatives to expression systems based on bacteria, yeast, insect, or mammalian cells. The free-floating aquatic plants of the Lemnaceae family (duckweed) have compact architecture and can be vegetatively propagated on low-cost nutrient solutions in aseptic conditions. These features provide an economically feasible opportunity for duckweed-based production of high-value products via transient expression of recombinant products in fully contained, controlled, aseptic and bio-safe conditions in accordance with the requirements for pharmaceutical manufacturing and environmental biosafety. Here, we demonstrated Agrobacterium-mediated high-yield transient expression of a reporter green fluorescent protein using deconstructed vectors based on potato virus X and sweet potato leaf curl virus, as well as conventional binary vectors, in two representatives of the Lemnaceae (Spirodela polyrhiza and Landoltia punctata). Aseptically cultivated duckweed populations yielded reporter protein accumulation of >1 mg/g fresh biomass, when the protein was expressed from a deconstructed potato virus X-based vector, which is capable of replication and cell-to-cell movement of the replicons in duckweed. The expression efficiency demonstrated here places duckweed among the most efficient host organisms for plant-based transient expression systems, with the additional benefits of easy scale-up and full containment.
Keywords: duckweed; expression vector; plant expression system; recombinant proteins; transient expression.
Copyright © 2021 Peterson, Kishchenko, Zhou, Vasylenko, Giritch, Sun, Borisjuk and Kuchuk.
Conflict of interest statement
AG is employed by Nomad Bioscience GmbH. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
The "Duckweed Dip": Aquatic Spirodela polyrhiza Plants Can Efficiently Uptake Dissolved, DNA-Wrapped Carbon Nanotubes from Their Environment for Transient Gene Expression.ACS Synth Biol. 2024 Feb 16;13(2):687-691. doi: 10.1021/acssynbio.3c00620. Epub 2023 Dec 21. ACS Synth Biol. 2024. PMID: 38127817 Free PMC article.
-
Growth Promotion of Giant Duckweed Spirodela polyrhiza (Lemnaceae) by Ensifer sp. SP4 Through Enhancement of Nitrogen Metabolism and Photosynthesis.Mol Plant Microbe Interact. 2022 Jan;35(1):28-38. doi: 10.1094/MPMI-06-21-0157-R. Epub 2021 Dec 17. Mol Plant Microbe Interact. 2022. PMID: 34622686
-
Development of a New Marker System for Identification of Spirodela polyrhiza and Landoltia punctata.Int J Genomics. 2017;2017:5196763. doi: 10.1155/2017/5196763. Epub 2017 Jan 12. Int J Genomics. 2017. PMID: 28168191 Free PMC article.
-
Literature review on duckweed toxicity testing.Environ Res. 1990 Jun;52(1):7-22. doi: 10.1016/s0013-9351(05)80147-1. Environ Res. 1990. PMID: 2190824 Review.
-
The Developmental Cycle of Spirodela polyrhiza Turions: A Model for Turion-Based Duckweed Overwintering?Plants (Basel). 2024 Oct 26;13(21):2993. doi: 10.3390/plants13212993. Plants (Basel). 2024. PMID: 39519914 Free PMC article. Review.
Cited by
-
Biodiversity of Duckweed (Lemnaceae) in Water Reservoirs of Ukraine and China Assessed by Chloroplast DNA Barcoding.Plants (Basel). 2022 May 30;11(11):1468. doi: 10.3390/plants11111468. Plants (Basel). 2022. PMID: 35684242 Free PMC article.
-
The "Duckweed Dip": Aquatic Spirodela polyrhiza Plants Can Efficiently Uptake Dissolved, DNA-Wrapped Carbon Nanotubes from Their Environment for Transient Gene Expression.ACS Synth Biol. 2024 Feb 16;13(2):687-691. doi: 10.1021/acssynbio.3c00620. Epub 2023 Dec 21. ACS Synth Biol. 2024. PMID: 38127817 Free PMC article.
-
Cryopreservation of Duckweed Genetic Diversity as Model for Long-Term Preservation of Aquatic Flowering Plants.Plants (Basel). 2023 Sep 18;12(18):3302. doi: 10.3390/plants12183302. Plants (Basel). 2023. PMID: 37765466 Free PMC article.
-
Genetic transformation of the oilseed crop camelina using immature zygotic embryos.Plant Methods. 2025 Mar 13;21(1):35. doi: 10.1186/s13007-025-01351-2. Plant Methods. 2025. PMID: 40075505 Free PMC article.
-
Duckweeds for Phytoremediation of Polluted Water.Plants (Basel). 2023 Jan 29;12(3):589. doi: 10.3390/plants12030589. Plants (Basel). 2023. PMID: 36771672 Free PMC article. Review.
References
-
- Appenroth K.-J., Teller S., Horn M. (1996). Photophysiology of Turion Formation and Germination in Spirodela Polyrhiza . Biol. Plant. 38. 10.1007/BF02879642 - DOI
LinkOut - more resources
Full Text Sources

