Characterization of Recombinant Chimpanzee Adenovirus C68 Low and High-Density Particles: Impact on Determination of Viral Particle Titer
- PMID: 34805110
- PMCID: PMC8599148
- DOI: 10.3389/fbioe.2021.753480
Characterization of Recombinant Chimpanzee Adenovirus C68 Low and High-Density Particles: Impact on Determination of Viral Particle Titer
Abstract
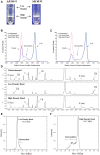
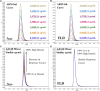
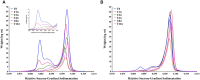
We observed differential infectivity and product yield between two recombinant chimpanzee adenovirus C68 constructs whose primary difference was genome length. To determine a possible reason for this outcome, we characterized the proportion and composition of the empty and packaged capsids. Both analytical ultracentrifugation (AUC) and differential centrifugation sedimentation (DCS, a rapid and quantitative method for measuring adenoviral packaging variants) were employed for an initial assessment of genome packaging and showed multiple species whose abundance deviated between the virus builds but not manufacturing campaigns. Identity of the packaging variants was confirmed by charge detection mass spectrometry (CDMS), the first known application of this technique to analyze adenovirus. The empty and packaged capsid populations were separated via preparative ultracentrifugation and then combined into a series of mixtures. These mixtures showed the oft-utilized denaturing A260 adenoviral particle titer method will underestimate the actual particle titer by as much as three-fold depending on the empty/full ratio. In contrast, liquid chromatography with fluorescence detection proves to be a superior viral particle titer methodology.
Keywords: AEX-HPLC; adenovirus; analytical ultracentrifugation; charge detection mass spectrometry; differential centrifugation sedimentation; low density viral particles; non-human primate; viral particle titer.
Copyright © 2021 Mullins, Powers, Zobel, Clawson, Barnes, Draper, Zou, Binder, Dai, Zhang, Friese, Runnels, Jarrold and Thompson.
Conflict of interest statement
Authors EKM, TWP, JZ, KMC, QC, KZ, OF, HAR, LCT and JJB were employed by Pfizer Inc. Author BED was employed by Megadalton Solutions. Author SD was employed by Nektar Therapeutics. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Identification of Recombinant Chimpanzee Adenovirus C68 Degradation Products Detected by AEX-HPLC.Front Bioeng Biotechnol. 2022 Apr 5;10:753481. doi: 10.3389/fbioe.2022.753481. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35449595 Free PMC article.
-
Measurement of Adenovirus-Based Vector Heterogeneity.J Pharm Sci. 2023 Apr;112(4):974-984. doi: 10.1016/j.xphs.2022.12.012. Epub 2022 Dec 21. J Pharm Sci. 2023. PMID: 36563855 Free PMC article.
-
Determination of particle heterogeneity and stability of recombinant adenovirus by analytical ultracentrifugation in CsCl gradients.J Pharm Sci. 2008 Feb;97(2):746-63. doi: 10.1002/jps.21008. J Pharm Sci. 2008. PMID: 17593554
-
Empty capsids in column-purified recombinant adenovirus preparations.Hum Gene Ther. 2001 Oct 10;12(15):1923-36. doi: 10.1089/104303401753153974. Hum Gene Ther. 2001. PMID: 11589834
-
Developing an Anion Exchange Chromatography Assay for Determining Empty and Full Capsid Contents in AAV6.2.Mol Ther Methods Clin Dev. 2019 Sep 26;15:257-263. doi: 10.1016/j.omtm.2019.09.006. eCollection 2019 Dec 13. Mol Ther Methods Clin Dev. 2019. PMID: 31720304 Free PMC article.
Cited by
-
Charge detection mass spectrometry for the analysis of viruses and virus-like particles.Essays Biochem. 2023 Mar 29;67(2):315-323. doi: 10.1042/EBC20220101. Essays Biochem. 2023. PMID: 36062529 Free PMC article.
-
Identification of Recombinant Chimpanzee Adenovirus C68 Degradation Products Detected by AEX-HPLC.Front Bioeng Biotechnol. 2022 Apr 5;10:753481. doi: 10.3389/fbioe.2022.753481. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35449595 Free PMC article.
-
Charge Detection Mass Spectrometry Enables Molecular Characterization of Nucleic Acid Nanoparticles.ACS Nano. 2024 Aug 27;18(34):23301-23309. doi: 10.1021/acsnano.4c06313. Epub 2024 Aug 16. ACS Nano. 2024. PMID: 39151088
References
LinkOut - more resources
Full Text Sources

