Superovulation Does Not Alter Calcium Oscillations Following Fertilization
- PMID: 34805168
- PMCID: PMC8601230
- DOI: 10.3389/fcell.2021.762057
Superovulation Does Not Alter Calcium Oscillations Following Fertilization
Abstract
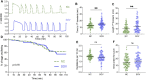
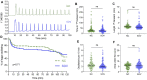
Superovulation is a common approach to maximize the number of eggs available for either clinical assisted reproductive technologies or experimental animal studies. This procedure provides supraphysiological amounts of gonadotropins to promote continued growth and maturation of ovarian follicles that otherwise would undergo atresia. There is evidence in mice, cows, sheep, and humans that superovulation has a detrimental impact on the quality of the resulting ovulated eggs or embryos. Here we tested the hypothesis that eggs derived from superovulation have a reduced capacity to support calcium oscillations, which are a critical factor in the success of embryo development. Eggs were obtained from mice that were either naturally cycling or underwent a standard superovulation protocol. The eggs were either parthenogenetically activated using strontium or fertilized in vitro while undergoing monitoring of calcium oscillatory patterns. Following parthenogenetic activation, superovulated eggs had a slightly delayed onset and longer duration of the first calcium transient, but no differences in oscillation persistence, frequency, or total calcium signal. However, in vitro fertilized superovulated eggs had no differences in any of these measures of calcium oscillatory behavior relative to spontaneously ovulated eggs. These findings indicate that although subtle differences in calcium signaling can be detected following parthenogenetic activation, superovulation does not disrupt physiological calcium signaling at fertilization, supporting the use of this method for both clinical and experimental purposes.
Keywords: calcium oscillations; egg activation; mouse; oocyte; superovulation.
Copyright © 2021 Savy, Stein, Shi and Williams.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Baart E. B., Martini E., Eijkemans M. J., Van Opstal D., Beckers N. G. M., Verhoeff A., et al. (2007). Milder Ovarian Stimulation for In-Vitro Fertilization Reduces Aneuploidy in the Human Preimplantation Embryo: a Randomized Controlled Trial. Hum. Reprod. 22, 980–988. 10.1093/humrep/del484 - DOI - PubMed
-
- Callesen H., Greve T., Hyttel P. (1986). Preovulatory Endocrinology and Oocyte Maturation in Superovulated Cattle. Theriogenology 25, 71–86. 10.1016/0093-691X(86)90184-6 - DOI
LinkOut - more resources
Full Text Sources

