Mitochondrial Defects in Fibroblasts of Pathogenic MAPT Patients
- PMID: 34805172
- PMCID: PMC8595217
- DOI: 10.3389/fcell.2021.765408
Mitochondrial Defects in Fibroblasts of Pathogenic MAPT Patients
Abstract
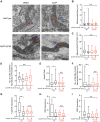
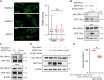
Mutations in MAPT gene cause multiple neurological disorders, including frontal temporal lobar degeneration and parkinsonism. Increasing evidence indicates impaired mitochondrial homeostasis and mitophagy in patients and disease models of pathogenic MAPT. Here, using MAPT patients' fibroblasts as a model, we report that disease-causing MAPT mutations compromise early events of mitophagy. By employing biochemical and mitochondrial assays we discover that upon mitochondrial depolarization, the recruitment of LRRK2 and Parkin to mitochondria and degradation of the outer mitochondrial membrane protein Miro1 are disrupted. Using high resolution electron microscopy, we reveal that the contact of mitochondrial membranes with ER and cytoskeleton tracks is dissociated following mitochondrial damage. This membrane dissociation is blocked by a pathogenic MAPT mutation. Furthermore, we provide evidence showing that tau protein, which is encoded by MAPT gene, interacts with Miro1 protein, and this interaction is abolished by pathogenic MAPT mutations. Lastly, treating fibroblasts of a MAPT patient with a small molecule promotes Miro1 degradation following depolarization. Altogether, our results show molecular defects in a peripheral tissue of patients and suggest that targeting mitochondrial quality control may have a broad application for future therapeutic intervention.
Keywords: ER; FTLD; MAPT; Miro; mitochondria; mitophagy; parkinsonism; tau.
Copyright © 2021 Bharat, Hsieh and Wang.
Conflict of interest statement
XW is a co-founder, adviser, and shareholder of AcureX Therapeutics Inc., and a shareholder of Mitokinin Inc. C-HH is a shareholder of AcureX Therapeutics Inc. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Bonifati V. (2002). Deciphering Parkinson’s disease–PARK8. Lancet Neurol. 1:83. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

