Challenges During Review of COVID-19 Research Proposals: Experience of Faculty of Medicine, Ain Shams University Research Ethics Committee, Egypt
- PMID: 34805197
- PMCID: PMC8596560
- DOI: 10.3389/fmed.2021.715796
Challenges During Review of COVID-19 Research Proposals: Experience of Faculty of Medicine, Ain Shams University Research Ethics Committee, Egypt
Abstract
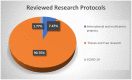
The COVID-19 pandemic resulted in an overwhelming increase in research studies submitted to research ethics committees (RECs) presenting many ethical challenges. This article aims to report the challenges encountered during review of COVID-19 research and the experience of the Faculty of Medicine, Ain Shams University Research Ethics Committee (FMASU REC). From April 10, 2020, until October 13, 2020, the FMASU REC reviewed 98 COVID-19 research protocols. This article addressed the question of how to face an overwhelming amount of research submitted to the REC while applying the required ethical principles. Ethical challenges included a new accelerated mode of review, online meetings, balance of risks vs. benefits, measures to mitigate risks, co-enrolment in different studies, protection of a vulnerable COVID-19 population, accelerated decisions, online research, how to handle informed consent during the pandemic, and justification of placebo arm.
Keywords: COVID-19 research; Faculty of Medicine Ain Shams University; accelerated review; ethical challenges; research ethics committees.
Copyright © 2021 Marzouk, Sharawy, Nakhla, El Hodhod, Gadallah, El-Shalakany, Elwakil, Moussa, Ismail and Tash.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Sisi Passes Law Regulating Clinical Medical Research in Egypt . Egypt: Independent Gazette (2020). Available online at: https://egyptindependent.com/sisi-passes-law-regulating-clinical-medical... (accessed February 28, 2021)
-
- Available, online at: https://clinicaltrials.gov/ct2/results?cond=COVID-19 (accessed March 3, 2021)
-
- Guidance for Managing Ethical Issues in Infectious Disease Outbreaks. Geneva: World Health Organization; (2016). Available online at: https://apps.who.int/iris/bitstream/handle/10665/250580/9789241549837-en... (accessed November 10, 2020)
-
- Ethical Standards for Research During Public Health Emergencies: Distilling Existing Guidance to Support COVID-19 R&D . Geneva: World Health Organization; (2020). Available online at: https://apps.who.int/iris/bitstream/handle/10665/331507/WHO-RFH-20.1-eng... (accessed November 10, 2020)
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous