Spatial and Temporal Analysis of Sodium-Ion Batteries
- PMID: 34805527
- PMCID: PMC8593912
- DOI: 10.1021/acsenergylett.1c01868
Spatial and Temporal Analysis of Sodium-Ion Batteries
Abstract
As a promising alternative to the market-leading lithium-ion batteries, low-cost sodium-ion batteries (SIBs) are attractive for applications such as large-scale electrical energy storage systems. The energy density, cycling life, and rate performance of SIBs are fundamentally dependent on dynamic physiochemical reactions, structural change, and morphological evolution. Therefore, it is essential to holistically understand SIBs reaction processes, degradation mechanisms, and thermal/mechanical behaviors in complex working environments. The recent developments of advanced in situ and operando characterization enable the establishment of the structure-processing-property-performance relationship in SIBs under operating conditions. This Review summarizes significant recent progress in SIBs exploiting in situ and operando techniques based on X-ray and electron analyses at different time and length scales. Through the combination of spectroscopy, imaging, and diffraction, local and global changes in SIBs can be elucidated for improving materials design. The fundamental principles and state-of-the-art capabilities of different techniques are presented, followed by elaborative discussions of major challenges and perspectives.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





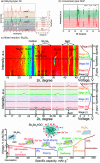
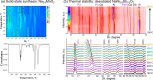
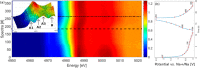
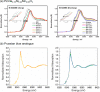
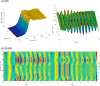


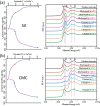

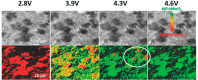
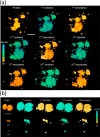

References
-
- Whittingham M. S.; Gamble F. R. The Lithium Intercalates of the Transition Metal Dichalcogenides. Mater. Res. Bull. 1975, 10 (5), 363–371. 10.1016/0025-5408(75)90006-9. - DOI
-
- Fouassier C.; Delmas C.; Hagenmuller P. Evolution structurale et proprietes physiques des phases AXMO2 (A = Na, K, M = Cr, Mn, Co) (x ⩽ 1). Mater. Res. Bull. 1975, 10 (6), 443–449. 10.1016/0025-5408(75)90166-X. - DOI
-
- Braconnier J.-J.; Delmas C.; Fouassier C.; Hagenmuller P. Comportement electrochimique des phases NaxCoO2. Mater. Res. Bull. 1980, 15 (12), 1797–1804. 10.1016/0025-5408(80)90199-3. - DOI
-
- Slater M. D.; Kim D.; Lee E.; Johnson C. S. Sodium-Ion Batteries. Adv. Funct. Mater. 2013, 23 (8), 947–958. 10.1002/adfm.201200691. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
