3D Printing of Tricalcium Phosphate/Poly Lactic-co-glycolic Acid Scaffolds Loaded with Carfilzomib for Treating Critical-sized Rabbit Radial Bone Defects
- PMID: 34805594
- PMCID: PMC8600297
- DOI: 10.18063/ijb.v7i4.405
3D Printing of Tricalcium Phosphate/Poly Lactic-co-glycolic Acid Scaffolds Loaded with Carfilzomib for Treating Critical-sized Rabbit Radial Bone Defects
Abstract
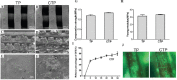
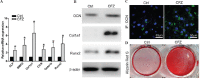
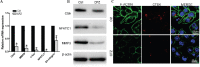
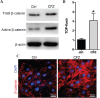
The rapid development of scaffold-based bone tissue engineering strongly relies on the fabrication of advanced scaffolds and the use of newly discovered functional drugs. As the creation of new drugs and their clinical approval often cost a long time and billions of U.S. dollars, producing scaffolds loaded with repositioned conventional drugs whose biosafety has been verified clinically to treat critical-sized bone defect has gained increasing attention. Carfilzomib (CFZ), an approved clinical proteasome inhibitor with a much fewer side effects, is used to replace bortezomib to treat multiple myeloma. It is also reported that CFZ could enhance the activity of alkaline phosphatase and increase the expression of osteogenic transcription factors. With the above consideration, in this study, a porous CFZ/β-tricalcium phosphate/poly lactic-co-glycolic acid scaffold (designated as "cytidine triphosphate [CTP]") was produced through cryogenic three-dimensional (3D) printing. The hierarchically porous CTP scaffolds were mechanically similar to human cancellous bone and can provide a sustained CFZ release. The implantation of CTP scaffolds into critical-sized rabbit radius bone defects improved the growth of new blood vessels and significantly promoted new bone formation. To the best of our knowledge, this is the first work that shows that CFZ-loaded scaffolds could treat nonunion of bone defect by promoting osteogenesis and angiogenesis while inhibiting osteoclastogenesis, through the activation of the Wnt/β-catenin signaling. Our results suggest that the loading of repositioned drugs with effective osteogenesis capability in advanced bone tissue engineering scaffold is a promising way to treat critical-sized defects of a long bone.
Keywords: Bone defect; Bone regeneration; Carfilzomib; Cryogenic 3D printing; β-tricalcium phosphate.
Copyright: © 2021 Li, et al.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures



Similar articles
-
Fabrication and Application of Novel Porous Scaffold in Situ-Loaded Graphene Oxide and Osteogenic Peptide by Cryogenic 3D Printing for Repairing Critical-Sized Bone Defect.Molecules. 2019 Apr 28;24(9):1669. doi: 10.3390/molecules24091669. Molecules. 2019. PMID: 31035401 Free PMC article.
-
Cryogenic 3D Printing of w/o Pickering Emulsions Containing Bifunctional Drugs for Producing Hierarchically Porous Bone Tissue Engineering Scaffolds with Antibacterial Capability.Int J Mol Sci. 2022 Aug 27;23(17):9722. doi: 10.3390/ijms23179722. Int J Mol Sci. 2022. PMID: 36077120 Free PMC article.
-
3D-printed HA15-loaded β-Tricalcium Phosphate/Poly (Lactic-co-glycolic acid) Bone Tissue Scaffold Promotes Bone Regeneration in Rabbit Radial Defects.Int J Bioprint. 2021 Jan 20;7(1):317. doi: 10.18063/ijb.v7i1.317. eCollection 2021. Int J Bioprint. 2021. PMID: 33585714 Free PMC article.
-
Three-dimensional (3D) printed scaffold and material selection for bone repair.Acta Biomater. 2019 Jan 15;84:16-33. doi: 10.1016/j.actbio.2018.11.039. Epub 2018 Nov 24. Acta Biomater. 2019. PMID: 30481607 Review.
-
Milestones in Mandibular Bone Tissue Engineering: A Systematic Review of Large Animal Models and Critical-Sized Defects.J Clin Med. 2025 Apr 15;14(8):2717. doi: 10.3390/jcm14082717. J Clin Med. 2025. PMID: 40283548 Free PMC article. Review.
Cited by
-
Treatment of Bone Defects and Nonunion via Novel Delivery Mechanisms, Growth Factors, and Stem Cells: A Review.ACS Biomater Sci Eng. 2024 Dec 9;10(12):7314-7336. doi: 10.1021/acsbiomaterials.4c01279. Epub 2024 Nov 11. ACS Biomater Sci Eng. 2024. PMID: 39527574 Free PMC article. Review.
-
3D-printed vascularized biofunctional scaffold for bone regeneration.Int J Bioprint. 2023 Mar 8;9(3):702. doi: 10.18063/ijb.702. eCollection 2023. Int J Bioprint. 2023. PMID: 37273991 Free PMC article.
-
369Fabrication of 3D gel-printed β-tricalcium phosphate/titanium dioxide porous scaffolds for cancellous bone tissue engineering.Int J Bioprint. 2023 Jan 19;9(2):673. doi: 10.18063/ijb.v9i2.673. eCollection 2023. Int J Bioprint. 2023. PMID: 37065658 Free PMC article.
-
Analysis of the CPZ/Wnt4 osteogenic pathway for high-bonding-strength composite-coated magnesium scaffolds through transcriptomics.Mater Today Bio. 2024 Sep 8;28:101234. doi: 10.1016/j.mtbio.2024.101234. eCollection 2024 Oct. Mater Today Bio. 2024. PMID: 39309165 Free PMC article.
References
-
- Sharif F, Ur Rehman I, Muhammad N, et al. Dental Materials for Cleft Palate Repair. Mater Sci Eng C Mater Biol Appl. 2016;61:1018–28. https://doi.org/10.1016/j.msec.2015.12.019. - PubMed
-
- Marx RE. Bone and Bone Graft Healing. Oral Maxillofac Surg Clin North Am. 2007;19:455–66. - PubMed
-
- Agarwal R, García AJ. Biomaterial Strategies for Engineering Implants for Enhanced Osseointegration and Bone Repair. Adv Drug Deliv Rev. 2015;94:53–62. https://doi.org/10.1016/j.addr.2015.03.013. - PMC - PubMed
-
- Pilliar RM, Filiaggi MJ, Wells JD, et al. Porous Calcium Polyphosphate Scaffolds for Bone Substitute Applications-In Vitro Characterization. Biomaterials. 2001;22:963–72. https://doi.org/10.1016/s0142-9612(00)00261-1. - PubMed
-
- Lai Y, Li Y, Cao H, et al. Osteogenic Magnesium Incorporated into PLGA/TCP Porous Scaffold by 3D Printing for Repairing Challenging Bone Defect. Biomaterials. 2019;197:207–19. https://doi.org/10.1016/j.biomaterials.2019.01.013. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
