Efficacy of Evocalcet in Previously Cinacalcet-Treated Secondary Hyperparathyroidism Patients
- PMID: 34805635
- PMCID: PMC8589700
- DOI: 10.1016/j.ekir.2021.08.020
Efficacy of Evocalcet in Previously Cinacalcet-Treated Secondary Hyperparathyroidism Patients
Erratum in
-
Corrigendum to "Efficacy of evocalcet in previously cinacalcet-treated secondary hyperparathyroidism patients," [Kidney Int. Rep. 2021; https://doi.org/10.1016/j.ekir.2021.08.020].Kidney Int Rep. 2021 Nov 5;6(11):2936-2937. doi: 10.1016/j.ekir.2021.09.011. eCollection 2021 Nov. Kidney Int Rep. 2021. PMID: 34826317 Free PMC article.
Abstract
Introduction: Evocalcet is a recently approved calcimimetic agent for secondary hyperparathyroidism (SHPT). In this study, the efficacy and safety of once-daily oral evocalcet were evaluated in patients without prior cinacalcet use (nonusers) and previously treated patients (users).
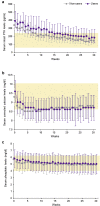
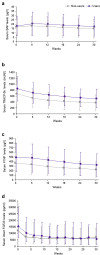
Methods: This post hoc analysis of a previous phase III head-to-head comparison study included SHPT patients treated with evocalcet with or without prior cinacalcet use. Endpoints included trends in the median intact and whole parathyroid hormone (PTH), mean corrected calcium, phosphate, and bone metabolic markers, and whole-to-intact PTH ratios throughout the 30-week study period; proportions of patients achieving target intact PTH, corrected calcium, and phosphate at weeks 28 to 30; and adverse drug reactions (ADRs).
Results: This study included 127 nonusers and 190 users with significant differences in age; duration of dialysis; use of intravenous vitamin D receptor activators; levels of intact PTH, corrected calcium, tartrate-resistant acid phosphatase 5b, procollagen type 1 N-terminal-propeptide; and largest parathyroid gland volume (P < 0.05 for all characteristics) between 2 groups at baseline. Users required higher evocalcet dosages than nonusers. Similar efficacy results were found in the 2 groups except for a significantly higher proportion of nonusers achieving the intact PTH target (81.6% vs 67.1%, difference [95% confidence interval], -14.5% [-24.59, -3.34]), and a significant reduction in largest parathyroid gland volume from week 0 to week 30 (-120.6 [567.2] mm3, P = 0.043). No difference was found in ADRs between the 2 groups.
Conclusion: Treatment with evocalcet is effective and safe irrespective of prior cinacalcet treatment in SHPT patients.
Keywords: calcium; cinacalcet; evocalcet; parathyroid gland volume; parathyroid hormone; secondary hyperparathyroidism.
© 2021 International Society of Nephrology. Published by Elsevier Inc.
Figures

Similar articles
-
Predictive factors requiring high-dose evocalcet in hemodialysis patients with secondary hyperparathyroidism.PLoS One. 2022 Dec 13;17(12):e0279078. doi: 10.1371/journal.pone.0279078. eCollection 2022. PLoS One. 2022. PMID: 36512619 Free PMC article. Clinical Trial.
-
Head-to-head comparison of the new calcimimetic agent evocalcet with cinacalcet in Japanese hemodialysis patients with secondary hyperparathyroidism.Kidney Int. 2018 Oct;94(4):818-825. doi: 10.1016/j.kint.2018.05.013. Epub 2018 Jul 24. Kidney Int. 2018. PMID: 30049473 Clinical Trial.
-
Efficacy and Safety of Evocalcet Evaluated by Dialysate Calcium Concentration in Patients with Secondary Hyperparathyroidism Undergoing Hemodialysis.Int J Nephrol Renovasc Dis. 2020 May 12;13:97-106. doi: 10.2147/IJNRD.S243210. eCollection 2020. Int J Nephrol Renovasc Dis. 2020. PMID: 32494184 Free PMC article.
-
Development of evocalcet for unmet needs among calcimimetic agents.Expert Rev Endocrinol Metab. 2020 Sep;15(5):299-310. doi: 10.1080/17446651.2020.1780911. Epub 2020 Jun 18. Expert Rev Endocrinol Metab. 2020. PMID: 32552012 Review.
-
Comparative Effectiveness of Calcimimetic Agents for Secondary Hyperparathyroidism in Adults: A Systematic Review and Network Meta-analysis.Am J Kidney Dis. 2020 Sep;76(3):321-330. doi: 10.1053/j.ajkd.2020.02.439. Epub 2020 May 28. Am J Kidney Dis. 2020. PMID: 32475604
Cited by
-
Long-Term Efficacy and Safety of Upacicalcet in Japanese Hemodialysis Patients with Secondary Hyperparathyroidism: Open-Label 52-Week Study.Am J Nephrol. 2025;56(1):70-84. doi: 10.1159/000541493. Epub 2024 Sep 19. Am J Nephrol. 2025. PMID: 39299219 Free PMC article. Clinical Trial.
-
Comparison of the Oral Calcimimetics Evocalcet and Cinacalcet in East Asian Patients on Hemodialysis with Secondary Hyperparathyroidism.Kidney Int Rep. 2023 Aug 29;8(11):2294-2306. doi: 10.1016/j.ekir.2023.08.034. eCollection 2023 Nov. Kidney Int Rep. 2023. PMID: 38025238 Free PMC article.
-
Effectiveness of calcimimetics on fractures in dialysis patients with secondary hyperparathyroidism: meta-analysis of randomized trials.J Bone Miner Metab. 2024 May;42(3):316-325. doi: 10.1007/s00774-024-01500-y. Epub 2024 Mar 27. J Bone Miner Metab. 2024. PMID: 38536478
-
Evaluation of the Interaction of Cinacalcet with Calf Thymus dsDNA: Use of Electrochemical, Spectrofluorimetric, and Molecular Docking Methods.Biosensors (Basel). 2022 Apr 27;12(5):278. doi: 10.3390/bios12050278. Biosensors (Basel). 2022. PMID: 35624577 Free PMC article.
References
-
- Cunningham J., Locatelli F., Rodriguez M. Secondary hyperparathyroidism: pathogenesis, disease progression, and therapeutic options. Clin J Am Soc Nephrol. 2011;6:913–921. - PubMed
-
- Komaba H., Kakuta T., Fukagawa M. Diseases of the parathyroid gland in chronic kidney disease. Clin Exp Nephrol. 2011;15:797–809. - PubMed
-
- Ketteler M., Block G.A., Evenepoel P., et al. Executive summary of the 2017 KDIGO Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Guideline Update: what’s changed and why it matters. Kidney Int. 2017;92:26–36. - PubMed
-
- Moe S., Drüeke T., Cunningham J., et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO) Kidney Int. 2006;69:1945–1953. - PubMed
-
- Fahrleitner-Pammer A., Herberth J., Browning S.R., et al. Bone markers predict cardiovascular events in chronic kidney disease. J Bone Miner Res. 2008;23:1850–1858. - PubMed
LinkOut - more resources
Full Text Sources

