Posterior cortical atrophy phenotypic heterogeneity revealed by decoding 18F-FDG-PET
- PMID: 34805993
- PMCID: PMC8600283
- DOI: 10.1093/braincomms/fcab182
Posterior cortical atrophy phenotypic heterogeneity revealed by decoding 18F-FDG-PET
Abstract
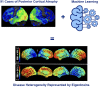
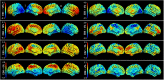
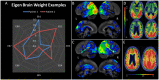
Posterior cortical atrophy is a neurodegenerative syndrome with a heterogeneous clinical presentation due to variable involvement of the left, right, dorsal and ventral parts of the visual system, as well as inconsistent involvement of other cognitive domains and systems. 18F-fluorodeoxyglucose (FDG)-PET is a sensitive marker for regional brain damage or dysfunction, capable of capturing the pattern of neurodegeneration at the single-participant level. We aimed to leverage these inter-individual differences on FDG-PET imaging to better understand the associations of heterogeneity of posterior cortical atrophy. We identified 91 posterior cortical atrophy participants with FDG-PET data and abstracted demographic, neurologic, neuropsychological and Alzheimer's disease biomarker data. The mean age at reported symptom onset was 59.3 (range: 45-72 years old), with an average disease duration of 4.2 years prior to FDG-PET scan, and a mean education of 15.0 years. Females were more common than males at 1.6:1. After standard preprocessing steps, the FDG-PET scans for the cohort were entered into an unsupervised machine learning algorithm which first creates a high-dimensional space of inter-individual covariance before performing an eigen-decomposition to arrive at a low-dimensional representation. Participant values ('eigenbrains' or latent vectors which represent principle axes of inter-individual variation) were then compared to the clinical and biomarker data. Eight eigenbrains explained over 50% of the inter-individual differences in FDG-PET uptake with left (eigenbrain 1) and right (eigenbrain 2) hemispheric lateralization representing 24% of the variance. Furthermore, eigenbrain-loads mapped onto clinical and neuropsychological data (i.e. aphasia, apraxia and global cognition were associated with the left hemispheric eigenbrain 1 and environmental agnosia and apperceptive prosopagnosia were associated with the right hemispheric eigenbrain 2), suggesting that they captured important axes of normal and abnormal brain function. We used NeuroSynth to characterize the eigenbrains through topic-based decoding, which supported the idea that the eigenbrains map onto a diverse set of cognitive functions. These eigenbrains captured important biological and pathophysiologic data (i.e. limbic predominant eigenbrain 4 patterns being associated with older age of onset compared to frontoparietal eigenbrain 7 patterns being associated with younger age of onset), suggesting that approaches that focus on inter-individual differences may be important to better understand the variability observed within a neurodegenerative syndrome like posterior cortical atrophy.
Keywords: FDG-PET; early-onset Alzheimer's disease; eigenvectors; posterior cortical atrophy; tau PET.
© The Author(s) (2021). Published by Oxford University Press on behalf of the Guarantors of Brain.
Figures

References
-
- Renner J, Burns J, Hou C, McKeel D, Storandt M, Morris J.. Progressive posterior cortical dysfunction: A clinicopathologic series. Neurology. 2004;63(7):1175–1180. - PubMed
-
- Tang-Wai DF, Graff-Radford N, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168–1174. - PubMed
-
- Benson DF, Davis RJ, Snyder BD.. Posterior cortical atrophy. Case reports. Arch Neurol. 1988;45(7):789–793. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
