Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis
- PMID: 34806056
- PMCID: PMC8592563
- DOI: 10.1016/S2666-5247(21)00267-6
Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis
Abstract
Background: Several SARS-CoV-2 variants of concern have been identified that partly escape serum neutralisation elicited by current vaccines. Studies have also shown that vaccines demonstrate reduced protection against symptomatic infection with SARS-CoV-2 variants. We explored whether in-vitro neutralisation titres remain predictive of vaccine protection from infection with SARS-CoV-2 variants.
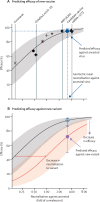
Methods: In this meta-analysis, we analysed published data from 24 identified studies on in-vitro neutralisation and clinical protection to understand the loss of neutralisation to existing SARS-CoV-2 variants of concern. We integrated the results of this analysis into our existing statistical model relating in-vitro neutralisation to protection (parameterised on data from ancestral virus infection) to estimate vaccine efficacy against SARS-CoV-2 variants. We also analysed data on boosting of vaccine responses and use the model to predict the impact of booster vaccination on protection against SARS-CoV-2 variants.
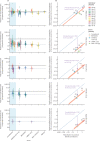
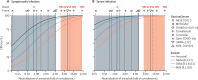
Findings: The neutralising activity against the ancestral SARS-CoV-2 was highly predictive of neutralisation of variants of concern. Decreases in neutralisation titre to the alpha (1·6-fold), beta (8·8-fold), gamma (3·5-fold), and delta (3·9-fold) variants (compared to the ancestral virus) were not significantly different between different vaccines. Neutralisation remained strongly correlated with protection from symptomatic infection with SARS-CoV-2 variants of concern (r S=0·81, p=0·0005) and the existing model remained predictive of vaccine efficacy against variants of concern once decreases in neutralisation to the variants of concern were incorporated. Modelling of predicted vaccine efficacy against variants over time suggested that protection against symptomatic infection might decrease below 50% within the first year after vaccination for some vaccines. Boosting of previously infected individuals with existing vaccines (which target ancestral virus) is predicted to provide a higher degree of protection from infection with variants of concern than primary vaccination schedules alone.
Interpretation: In-vitro neutralisation titres remain a correlate of protection from SARS-CoV-2 variants and modelling of the effects of waning immunity predicts a loss of protection to the variants after vaccination. However, booster vaccination with current vaccines should enable higher neutralisation to SARS-CoV-2 variants than is achieved with primary vaccination, which is predicted to provide robust protection from severe infection outcomes with the current SARS-CoV-2 variants of concern, at least in the medium term.
Funding: The National Health and Medical Research Council (Australia), the Medical Research Future Fund (Australia), and the Victorian Government.
© 2022 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license.
Conflict of interest statement
DSK is elected executive committee member of the New South Wales branch of the Australian and New Zealand Industrial and Applied Mathematics society. MPD is senior editor for eLife, for which he receives an annual retainer. All other authors declare no competing interests.
Figures


Comment in
-
Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants.Lancet Microbe. 2022 Mar;3(3):e167. doi: 10.1016/S2666-5247(21)00337-2. Epub 2021 Dec 23. Lancet Microbe. 2022. PMID: 34977829 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

