Fine Comparison of the Efficacy and Safety Between GB242 and Infliximab in Patients with Rheumatoid Arthritis: A Phase III Study
- PMID: 34806155
- PMCID: PMC8814292
- DOI: 10.1007/s40744-021-00396-8
Fine Comparison of the Efficacy and Safety Between GB242 and Infliximab in Patients with Rheumatoid Arthritis: A Phase III Study
Abstract
Introduction: This phase III trial (NCT04178850) evaluated the efficacy, safety, and immunogenicity of GB242, an infliximab biosimilar, vs. infliximab (Remicade®) reference product in patients with moderate-to-severe active rheumatoid arthritis (RA) combination with methotrexate (MTX) therapy.
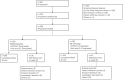
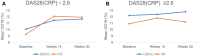
Methods: Patients were randomized in a 1:1 ratio to receive either GB242 or INF (3 mg/kg). Therapeutic equivalence of clinical response according to the American College of Rheumatology 20% (ACR20) response rate at week 30 was declared if the two-sided 95% CI for the treatment difference was within ± 14%. The comparison of GB242 with INF also included the proportion of patients achieving a week 30 ACR 50 response, ACR70 response, change in Disease Activity Score 28 (DAS28), as well as safety and immunogenicity.
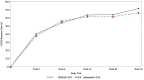
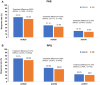
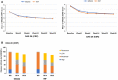
Results: A total of 570 subjects were randomized into GB242 (N = 285) or INF (N = 285) and 283 subjects in each group were analyzed. At week 30, the ACR20 was 62.54% for the GB242 group (95% CI 56.62-68.20%) and 56.89% for the INF group (95% CI 50.90-62.74%). The difference between the two groups was 5.65% with a 95% CI of - 2.48 to 13.74. ACR50 response was 37.12% for GB242 and 32.86% for INF at week 30. ACR70 response was 19.79% for GB242 and 16.96% for INF at week 30, respectively. The incidence of treatment-emergent adverse events was comparable (77.4% in GB242 vs. 80.2% in INF) and detection of antidrug antibodies (ADA) to infliximab up to week 30 (60.8% in GB242 vs. 59.4% in INF) was comparable.
Conclusions: GB242 demonstrated equivalent efficacy to INF at week 30. Moreover, GB242 was well tolerated, with a similar immunogenicity and safety profile comparable to INF.
Keywords: Biosimilar; GB242; Infliximab; Rheumatoid arthritis.
© 2021. The Author(s).
Figures
References
-
- Maini R, St Clair EW, Breedveld F, et al. Infliximab (chimeric anti-tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: a randomised phase III trial. ATTRACT Study Group. Lancet. 1999;354(9194):1932–1939. doi: 10.1016/S0140-6736(99)05246-0. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

