Phosphorylation of Lamin A/C at serine 22 modulates Nav 1.5 function
- PMID: 34806324
- PMCID: PMC8606869
- DOI: 10.14814/phy2.15121
Phosphorylation of Lamin A/C at serine 22 modulates Nav 1.5 function
Abstract
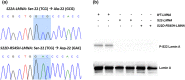
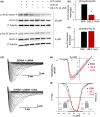
Variants in the LMNA gene, which encodes for Lamin A/C, are associated with cardiac conduction disease (CCD). We previously reported that Lamin A/C variants p.R545H and p.A287Lfs*193, which were identified in CCD patients, decreased peak INa in HEK-293 cells expressing Nav 1.5. Decreased peak INa in the cardiac conduction system could account for patients' atrioventricular block. We found that serine 22 (Ser 22) phosphorylation of Lamin A/C was decreased in the p.R545H variant and hypothesized that lamin phosphorylation modulated Nav 1.5 activity. To test this hypothesis, we assessed Nav 1.5 function in HEK-293 cells co-transfected with LMNA variants or treated with the small molecule LBL1 (lamin-binding ligand 1). LBL1 decreased Ser 22 phosphorylation by 65% but did not affect Nav 1.5 function. To test the complete loss of phosphorylation, we generated a version of LMNA with serine 22 converted to alanine 22 (S22A-LMNA); and a version of mutant R545H-LMNA that mimics phosphorylation via serine 22 to aspartic acid 22 substitution (S22D-R545H-LMNA). We found that S22A-LMNA inhibited Lamin-mediated activation of peak INa by 63% and shifted voltage-dependency of steady-state inactivation of Nav 1.5. Conversely, S22D-R545H-LMNA abolished the effects of mutant R545H-LMNA on voltage-dependency but not peak INa . We conclude that Lamin A/C Ser 22 phosphorylation can modulate Nav 1.5 function and contributes to the mechanism by which R545H-LMNA alters Nav 1.5 function. The differential impact of complete versus partial loss of Ser 22 phosphorylation suggests a threshold of phosphorylation that is required for full Nav 1.5 modulation. This is the first study to link Lamin A/C phosphorylation to Nav 1.5 function.
Keywords: Lamin phosphorylation; Nav1.5; cardiac conduction disease.
© 2021 The Authors. Physiological Reports published by Wiley Periodicals LLC on behalf of The Physiological Society and the American Physiological Society.
Conflict of interest statement
All authors declare no conflict of interest.
Figures



References
-
- Anselme, F. , Moubarak, G. , Savoure, A. , Godin, B. , Borz, B. , Drouin‐Garraud, V. , & Gay, A. (2013). Implantable cardioverter‐defibrillators in lamin A/C mutation carriers with cardiac conduction disorders. Heart Rhythm: the Official Journal of the Heart Rhythm Society, 10, 1492–1498. 10.1016/j.hrthm.2013.06.020 - DOI - PubMed
-
- Arbustini, E. , Pilotto, A. , Repetto, A. , Grasso, M. , Negri, A. , Diegoli, M. , Campana, C. , Scelsi, L. , Baldini, E. , Gavazzi, A. , & Tavazzi, L. (2002). Autosomal dominant dilated cardiomyopathy with atrioventricular block: A lamin A/C defect‐related disease. Journal of the American College of Cardiology, 39, 981–990. 10.1016/S0735-1097(02)01724-2 - DOI - PubMed
-
- Ben‐Harush, K. , Wiesel, N. , Frenkiel‐Krispin, D. , Moeller, D. , Soreq, E. , Aebi, U. , Herrmann, H. , Gruenbaum, Y. , & Medalia, O. (2009). The supramolecular organization of the C. elegans nuclear lamin filament. Journal of Molecular Biology, 386(5), 1392–1402. 10.1016/j.jmb.2008.12.024 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

