Directed Regeneration of Osteochondral Tissue by Hierarchical Assembly of Spatially Organized Composite Spheroids
- PMID: 34806336
- PMCID: PMC8787388
- DOI: 10.1002/advs.202103525
Directed Regeneration of Osteochondral Tissue by Hierarchical Assembly of Spatially Organized Composite Spheroids
Abstract
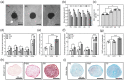
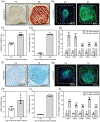
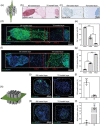
The use of engineered scaffolds or stem cells is investigated widely in the repair of injured musculoskeletal tissue. However, the combined regeneration of hierarchical osteochondral tissue remains a challenge due to delamination between cartilage and subchondral bone or difficulty in spatial control over differentiation of transplanted stem cells. Here, two types of composite spheroids are prepared using adipose-derived stem cells (hADSCs) and nanofibers coated with either transforming growth factor-β3 or bone morphogenetic growth factor-2 for chondrogenesis or osteogenesis, respectively. Each type of spheroid is then cultured within a 3D-printed microchamber in a spatially arranged manner to recapitulate the bilayer structure of osteochondral tissue. The presence of inductive factors regionally modulates in vitro chondrogenic or osteogenic differentiation of hADSCs within the biphasic construct without dedifferentiation. Furthermore, hADSCs from each spheroid proliferate and sprout and successfully connect the two layers mimicking the osteochondral interface without apertures. In vivo transplantation of the biphasic construct onto a femoral trochlear groove defect in rabbit knee joint results in 21.2 ± 2.8% subchondral bone volume/total volume and a cartilage score of 25.0 ± 3.7. The present approach can be an effective therapeutic platform to engineer complex tissue.
Keywords: 3D printing; composite spheroid; microchamber; osteochondral tissue regeneration; tissue engineering.
© 2021 The Authors. Advanced Science published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Vashisth P., Bellare J. R., Nanomed.: Nanotechnol., Biol. Med. 2018, 14, 1325.
-
- Yeung P., Zhang W., Wang X. N., Yan C. H., Chan B. P., Biomaterials 2018, 162, 1. - PubMed
-
- Kong J., Hwang I.‐W., Lee K., Adv. Mater. 2014, 26, 6275. - PubMed
-
- Yan Y., Chen H., Zhang H., Guo C., Yang K., Chen K., Cheng R., Qian N., Sandler N., Zhang Y. S., Shen H., Qi J., Cui W., Deng L., Biomaterials 2019, 190, 97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
