Mild Traumatic Brain Injury/Concussion Initiates an Atypical Astrocyte Response Caused by Blood-Brain Barrier Dysfunction
- PMID: 34806422
- PMCID: PMC8785769
- DOI: 10.1089/neu.2021.0204
Mild Traumatic Brain Injury/Concussion Initiates an Atypical Astrocyte Response Caused by Blood-Brain Barrier Dysfunction
Abstract
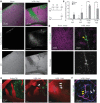
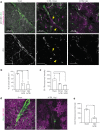
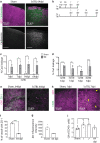
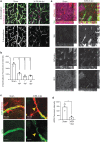
Mild traumatic brain injury/concussion (mTBI) accounts for 70-90% of all reported TBI cases and causes long-lasting neurological consequences in 10-40% of patients. Recent clinical studies revealed increased blood-brain barrier (BBB) permeability in mTBI patients, which correlated with secondary damage after mTBI. However, the cascade of cellular events initiated by exposure to blood-borne factors resulting in sustained damage is not fully understood. We previously reported that astrocytes respond atypically to mTBI, rapidly losing many proteins essential to their homeostatic function, while classic scar formation does not occur. Here, we tested the hypothesis that mTBI-induced BBB damage causes atypical astrocytes through exposure to blood-borne factors. Using an mTBI mouse model, two-photon imaging, an endothelial cell-specific genetic ablation approach, and serum-free primary astrocyte cultures, we demonstrated that areas with atypical astrocytes coincide with BBB damage and that exposure of astrocytes to plasma proteins is sufficient to initiate loss of astrocyte homeostatic proteins. Although mTBI resulted in frequent impairment of both physical and metabolic BBB properties and leakage of small-sized blood-borne factors, deposition of the coagulation factor fibrinogen or vessel rupture were rare. Surprisingly, even months after mTBI, BBB repair did not occur in areas with atypical astrocytes. Together, these findings implicate that even relatively small BBB disturbances are sustained long term, and render nearby astrocytes dysfunctional, likely at the cost of neuronal health and function.
Keywords: BBB repair; astrogliosis; brain injury; brain vasculature; repeated mild TBI; tight junctions.
Conflict of interest statement
No competing financial interests exist.
Figures


References
-
- Cassidy, J.D., Carroll, L.J., Peloso, P.M., Borg, J., von Holst, H., Holm, L., Kraus, J., and Coronado, V.G. (2004). Incidence, risk factors and prevention of mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. J. Rehabil. Med. 43, Suppl., 28–60. - PubMed
-
- Willer, B., and Leddy, J.J. (2006). Management of concussion and post-concussion syndrome. Curr. Treat. Options Neurol. 8, 415–426. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

