Site-Specific Cerebrospinal Fluid Tau Hyperphosphorylation in Response to Alzheimer's Disease Brain Pathology: Not All Tau Phospho-Sites are Hyperphosphorylated
- PMID: 34806603
- PMCID: PMC8842784
- DOI: 10.3233/JAD-210677
Site-Specific Cerebrospinal Fluid Tau Hyperphosphorylation in Response to Alzheimer's Disease Brain Pathology: Not All Tau Phospho-Sites are Hyperphosphorylated
Abstract
Background: Understanding patterns of association between CSF phosphorylated tau (p-tau) species and clinical disease severity will aid Alzheimer's disease (AD) diagnosis and treatment.
Objective: To evaluate changes in tau phosphorylation ratios to brain imaging (amyloid PET, [18F]GTP1 PET, and MRI) and cognition across clinical stages of AD in two different cohorts.
Methods: A mass spectrometry (MS)-based method was used to evaluate the relationship between p-tau/tau phosphorylation ratios on 11 sites in CSF and AD pathology measured by tau PET ([18F]GTP1) and amyloid PET ([18F]florbetapir or [18F]florbetaben). Cohort A included cognitively normal amyloid negative (n = 6) and positive (n = 5) individuals, and amyloid positive prodromal (n = 13), mild (n = 12), and moderate AD patients (n = 10); and Cohort B included amyloid positive prodromal (n = 24) and mild (n = 40) AD patients.
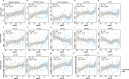
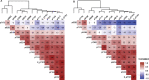
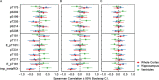
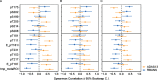
Results: In this cross-sectional analysis, we identified clusters of phosphosites with different profiles of phosphorylation ratios across stages of disease. Eight of 11 investigated sites were hyperphosphorylated and associated with SUVR measures from [18F]GTP1 and amyloid PET. Novel sites 111, 153, and 208 may be relevant biomarkers for AD diagnosis to complement tau hyperphosphorylation measures on previously established sites 181, 205, 217, and 231. Hypophosphorylation was detected on residues 175, 199, and 202, and was inversely associated with [18F]GTP1 and amyloid PET.
Conclusion: Hyperphosphorylated and hypophosphorylated forms of tau are associated with AD pathologies, and due to their different site-specific profiles, they may be used in combination to assist with staging of disease.
Keywords: Alzheimer’s disease; PET; [18F]GTP1; biomarker; cerebrospinal fluid; tau.
Conflict of interest statement
Authors’ disclosures available online (
Figures


References
-
- Nelson PT, Alafuzoff I, Bigio EH, Bouras C, Braak H, Cairns NJ, Castellani RJ, Crain BJ, Davies P, Del Tredici K, Duyckaerts C, Frosch MP, Haroutunian V, Hof PR, Hulette CM, Hyman BT, Iwatsubo T, Jellinger KA, Jicha GA, Kövari E, Kukull WA, Leverenz JB, Love S, Mackenzie IR, Mann DM, Masliah E, McKee AC, Montine TJ, Morris JC, Schneider JA, Sonnen JA, Thal DR, Trojanowski JQ, Troncoso JC, Wisniewski T, Woltjer RL, Beach TG (2012) Correlation of Alzheimer disease neuropathologic changes with cognitive status: A review of the literature. J Neuropathol Exp Neurol 71, 362–81. - PMC - PubMed
-
- Querfurth HW, LaFerla FM (2010) Alzheimer’s disease. N Engl J Med 362, 329–344. - PubMed
-
- Hanger DP, Anderton BH, Noble W (2009) Tau phosphorylation: The therapeutic challenge for neurodegenerative disease. Trends Mol Med 15, 112–9. - PubMed
-
- Augustinack JC, Schneider A, Mandelkow EM, Hyman BT (2002) Specific tau phosphorylation sites correlate with severity of neuronal cytopathology in Alzheimer’s disease. Acta Neuropathol 103, 26–3. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

