Benign tumors in TSC are amenable to treatment by GD3 CAR T cells in mice
- PMID: 34806651
- PMCID: PMC8663788
- DOI: 10.1172/jci.insight.152014
Benign tumors in TSC are amenable to treatment by GD3 CAR T cells in mice
Abstract
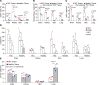
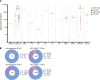
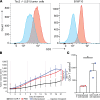
Mutations underlying disease in tuberous sclerosis complex (TSC) give rise to tumors with biallelic mutations in TSC1 or TSC2 and hyperactive mammalian target of rapamycin complex 1 (mTORC1). Benign tumors might exhibit de novo expression of immunogens, targetable by immunotherapy. As tumors may rely on ganglioside D3 (GD3) expression for mTORC1 activation and growth, we compared GD3 expression in tissues from patients with TSC and controls. GD3 was overexpressed in affected tissues from patients with TSC and also in aging Tsc2+/- mice. As GD3 overexpression was not accompanied by marked natural immune responses to the target molecule, we performed preclinical studies with GD3 chimeric antigen receptor (CAR) T cells. Polyfunctional CAR T cells were cytotoxic toward GD3-overexpressing targets. In mice challenged with Tsc2-/- tumor cells, CAR T cells substantially and durably reduced the tumor burden, correlating with increased T cell infiltration. We also treated aged Tsc2+/- heterozygous (>60 weeks) mice that carry spontaneous Tsc2-/- tumors with GD3 CAR or untransduced T cells and evaluated them at endpoint. Following CAR T cell treatment, the majority of mice were tumor free while all control animals carried tumors. The outcomes demonstrate a strong treatment effect and suggest that targeting GD3 can be successful in TSC.
Keywords: Antigen; Immunology; Immunotherapy; T cells; Therapeutics.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

