Emodin alleviates sepsis-mediated lung injury via inhibition and reduction of NF-kB and HMGB1 pathways mediated by SIRT1
- PMID: 34806822
- PMCID: PMC11896518
- DOI: 10.1002/kjm2.12476
Emodin alleviates sepsis-mediated lung injury via inhibition and reduction of NF-kB and HMGB1 pathways mediated by SIRT1
Abstract
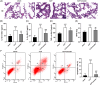
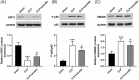
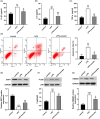
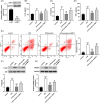
Inflammation plays an important role during sepsis, and excessive inflammation can result in organ damage, chronic inflammation, fibrosis, and scarring. The study aimed to investigate the specific mechanism of emodin by constructing in vivo and in vitro septic lung injury models via inhibition and reduction of NF-kB and high mobility group box 1 (HMGB1) pathways. A cecal ligation and puncture (CLP) model was built for adult male Sprague-Dawley rats. Concentrations of TNF-α, IL-1β, and IL-6 in bronchoalveolar lavage fluid were determined using commercially available ELISA kits. Hematoxylin and eosin staining was used for the right lung inferior lobes. Myeloperoxidase (MPO) activity of the lung tissue was detected by using the MPO kit. Murine alveolar epithelial cell line (MLE-12) cells were used for flow cytometry and Western blot to analyze the apoptosis rate and protein expression. Emodin significantly decreased CLP-induced cell apoptosis, upregulated expression of sirtuin 1 (SIRT1), and inhibited p-p65/p65 and HMGB1. In lipopolysaccharide (LPS) treated cell model, emodin treatment markedly decreased LPS-induced release of IL-1, IL-6, and tumor necrosis factor (TNF)-α, inhibited LPS-induced cell apoptosis and suppressed protein levels of P-P65/P65 and HMGB1. However, science of SIRT1 reversed the above effects by treatment of emodin. In summarize, this study found that emodin can alleviate sepsis-induced lung injury in vivo and in vitro through regulation of SIRT1.
Keywords: HMGB1; NF-kB; SIRT1; emodin; sepsis.
© 2021 The Authors. The Kaohsiung Journal of Medical Sciences published by John Wiley & Sons Australia on behalf of Kaohsiung Medical University.
Conflict of interest statement
All authors declare no conflict of interest.
Figures
Similar articles
-
Sepsis-induced acute lung injury in young rats is relieved by calycosin through inactivating the HMGB1/MyD88/NF-κB pathway and NLRP3 inflammasome.Int Immunopharmacol. 2021 Jul;96:107623. doi: 10.1016/j.intimp.2021.107623. Epub 2021 Apr 13. Int Immunopharmacol. 2021. PMID: 33857805
-
Alpha 2A-adrenoreceptor blockade improves sepsis-induced acute lung injury accompanied with depressed high mobility group box-1 levels in rats.Cytokine. 2012 Dec;60(3):639-45. doi: 10.1016/j.cyto.2012.08.002. Epub 2012 Sep 5. Cytokine. 2012. PMID: 22959885
-
Salidroside ameliorates sepsis-induced acute lung injury and mortality via downregulating NF-κB and HMGB1 pathways through the upregulation of SIRT1.Sci Rep. 2017 Sep 20;7(1):12026. doi: 10.1038/s41598-017-12285-8. Sci Rep. 2017. PMID: 28931916 Free PMC article.
-
Aloin Reduces HMGB1-Mediated Septic Responses and Improves Survival in Septic Mice by Activation of the SIRT1 and PI3K/Nrf2/HO-1 Signaling Axis.Am J Chin Med. 2019;47(3):613-633. doi: 10.1142/S0192415X19500320. Epub 2019 Apr 9. Am J Chin Med. 2019. PMID: 30966773
-
The protective effect of dexmedetomidine on LPS-induced acute lung injury through the HMGB1-mediated TLR4/NF-κB and PI3K/Akt/mTOR pathways.Mol Immunol. 2018 Feb;94:7-17. doi: 10.1016/j.molimm.2017.12.008. Epub 2017 Dec 11. Mol Immunol. 2018. PMID: 29241031
Cited by
-
Prognostic value of inflammatory cytokine detection for sepsis patients in ICU: a meta-analysis.Am J Transl Res. 2024 Jun 15;16(6):2612-2621. doi: 10.62347/NYLM7723. eCollection 2024. Am J Transl Res. 2024. PMID: 39006300 Free PMC article. Review.
-
The role and therapeutic potential of SIRTs in sepsis.Front Immunol. 2024 Apr 16;15:1394925. doi: 10.3389/fimmu.2024.1394925. eCollection 2024. Front Immunol. 2024. PMID: 38690282 Free PMC article. Review.
-
Research progress in the regulatory mechanism of silent information regulator 1 in sepsis (Review).Mol Med Rep. 2025 Aug;32(2):208. doi: 10.3892/mmr.2025.13573. Epub 2025 May 26. Mol Med Rep. 2025. PMID: 40417877 Free PMC article. Review.
-
Effect of emodin on acute lung injury: a meta-analysis of preclinical trials.BMC Pulm Med. 2024 Dec 2;24(1):596. doi: 10.1186/s12890-024-03406-x. BMC Pulm Med. 2024. PMID: 39623403 Free PMC article.
-
Sirt4 Overexpression Modulates the JAK2/STAT3 and PI3K/AKT/mTOR Axes to Alleviate Sepsis-Induced Acute Lung Injury.Cell Biochem Biophys. 2025 Jun;83(2):1785-1798. doi: 10.1007/s12013-024-01588-z. Epub 2024 Oct 14. Cell Biochem Biophys. 2025. PMID: 39400781
References
-
- Duyu M, Turkozkan C. Therapeutic plasma exchange in the pediatric intensive care unit: a single‐center 5‐year experience. Transfus Apher Sci. 2020;59(5):102959. - PubMed
-
- Guo R, Li Y, Han M, Liu J, Sun Y. Emodin attenuates acute lung injury in Cecal‐ligation and puncture rats. Int Immunopharmacol. 2020;85:106626. - PubMed
-
- de Castro R, Medeiros D, Prata‐Barbosa A, de Magalhães‐Barbosa M. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis‐associated organ dysfunction in children. Pediatr Crit Care Med. 2020;21(10):924–5. - PubMed
-
- Dong X, Zeng Y, Liu Y, You L, Yin X, Fu J, et al. Aloe‐emodin: a review of its pharmacology, toxicity, and pharmacokinetics. Phytother Res. 2020;34(2):270–81. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

