Accurate 57Fe Mössbauer Parameters from General Gaussian Basis Sets
- PMID: 34806886
- PMCID: PMC8675134
- DOI: 10.1021/acs.jctc.1c00722
Accurate 57Fe Mössbauer Parameters from General Gaussian Basis Sets
Abstract
The prediction of isomer shifts in 57Fe Mossbauer spectra is typically achieved by building calibration lines using the values of the density at the nuclear position. Using Slater-type orbital basis or large and specific Gaussian-type orbital basis has been thus far mandatory to achieve accurate predictions with density functional theory methods. In this work, we show that replacing the value of the density at the nucleus by the density integrated in a sphere of radius 0.06 au centered on the Fe nuclei yields excellent calibration lines (r2 = 0.976) with a high predictive power (q2 = 0.975, MAE = 0.055 mm·s-1) while using the conventional def2-TZVP basis set and X-ray geometrical parameters. Our data set comprises 69 57Fe-containing compounds and 103 signals. We also find B3LYP performing significantly better than the PW91 functional.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
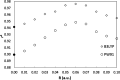
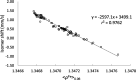

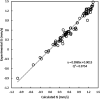

Similar articles
-
Calibration of DFT Functionals for the Prediction of Fe Mössbauer Spectral Parameters in Iron-Nitrosyl and Iron-Sulfur Complexes: Accurate Geometries Prove Essential.J Chem Theory Comput. 2011 Oct 11;7(10):3232-3247. doi: 10.1021/ct200187d. J Chem Theory Comput. 2011. PMID: 22039359 Free PMC article.
-
The prediction of Fe Mössbauer parameters by the density functional theory: a benchmark study.J Chem Theory Comput. 2010 Nov 9;6(12):3735-3749. doi: 10.1021/ct100398m. J Chem Theory Comput. 2010. PMID: 21258606 Free PMC article.
-
(57)Fe Mössbauer isomer shifts of heme protein model systems: electronic structure calculations.J Am Chem Soc. 2002 Jul 3;124(26):7829-39. doi: 10.1021/ja011583v. J Am Chem Soc. 2002. PMID: 12083937
-
On Predicting Mössbauer Parameters of Iron-Containing Molecules with Density-Functional Theory.J Chem Theory Comput. 2013 Nov 12;9(11):5004-5020. doi: 10.1021/ct4007585. Epub 2013 Oct 15. J Chem Theory Comput. 2013. PMID: 25821417 Free PMC article.
-
Density Functional Calculations for Prediction of 57Fe Mössbauer Isomer Shifts and Quadrupole Splittings in β-Diketiminate Complexes.ACS Omega. 2017 Jun 30;2(6):2594-2606. doi: 10.1021/acsomega.7b00595. Epub 2017 Jun 12. ACS Omega. 2017. PMID: 28691111 Free PMC article.
Cited by
-
Prediction of 57Fe Mössbauer Nuclear Quadrupole Splittings with Hybrid and Double-Hybrid Density Functionals.Int J Mol Sci. 2025 Mar 20;26(6):2821. doi: 10.3390/ijms26062821. Int J Mol Sci. 2025. PMID: 40141462 Free PMC article.
-
Debye Temperature Evaluation for Secondary Battery Cathode of α-SnxFe1-xOOH Nanoparticles Derived from the 57Fe- and 119Sn-Mössbauer Spectra.Int J Mol Sci. 2024 Feb 20;25(5):2488. doi: 10.3390/ijms25052488. Int J Mol Sci. 2024. PMID: 38473736 Free PMC article.
References
-
- Mössbauer R. L. Z. für Physik A Hadrons Nucl. 1958, 151, 124–143. 10.1007/bf01344210. - DOI
LinkOut - more resources
Full Text Sources

