Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease
- PMID: 34807243
- PMCID: PMC8609465
- DOI: 10.1001/jamaneurol.2021.4161
Amyloid-Related Imaging Abnormalities in 2 Phase 3 Studies Evaluating Aducanumab in Patients With Early Alzheimer Disease
Abstract
Importance: The EMERGE and ENGAGE phase 3 randomized clinical trials of aducanumab provide a robust data set to characterize amyloid-related imaging abnormalities (ARIA) that occur with treatment with aducanumab, an amyloid-β (Aβ)-targeting monoclonal antibody, in patients with mild cognitive impairment due to Alzheimer disease or mild Alzheimer disease dementia.
Objective: To describe the radiographic and clinical characteristics of ARIA that occurred in EMERGE and ENGAGE.
Design, setting, and participants: Secondary analysis of data from the EMERGE and ENGAGE trials, which were 2 double-blind, placebo-controlled, parallel-group, phase 3 randomized clinical trials that compared low-dose and high-dose aducanumab treatment with placebo among participants at 348 sites across 20 countries. Enrollment occurred from August 2015 to July 2018, and the trials were terminated early (March 21, 2019) based on a futility analysis. The combined studies consisted of a total of 3285 participants with Alzheimer disease who received 1 or more doses of placebo (n = 1087) or aducanumab (n = 2198; 2752 total person-years of exposure) during the placebo-controlled period. Primary data analyses were performed from November 2019 to July 2020, with additional analyses performed through July 2021.
Interventions: Participants were randomly assigned 1:1:1 to high-dose or low-dose intravenous aducanumab or placebo once every 4 weeks. Dose titration was used as a risk-minimization strategy.
Main outcomes and measures: Brain magnetic resonance imaging was used to monitor patients for ARIA; associated symptoms were reported as adverse events.
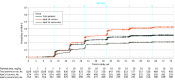
Results: Of 3285 included participants, the mean (SD) age was 70.4 (7.45) years; 1706 participants (52%) were female, 2661 (81%) had mild cognitive impairment due to Alzheimer disease, and 1777 (54%) used symptomatic medications for Alzheimer disease. A total of 764 participants from EMERGE and 709 participants from ENGAGE were categorized as withdrawn before study completion, most often owing to early termination of the study by the sponsor. Unless otherwise specified, all results represent analyses from the 10-mg/kg group. During the placebo-controlled period, 425 of 1029 patients (41.3%) experienced ARIA, with serious cases occurring in 14 patients (1.4%). ARIA-edema (ARIA-E) was the most common adverse event (362 of 1029 [35.2%]), and 263 initial events (72.7%) occurred within the first 8 doses of aducanumab; 94 participants (26.0%) with an event exhibited symptoms. Common associated symptoms among 103 patients with symptomatic ARIA-E or ARIA-H were headache (48 [46.6%]), confusion (15 [14.6%]), dizziness (11 [10.7%]), and nausea (8 [7.8%]). Incidence of ARIA-E was highest in aducanumab-treated participants who were apolipoprotein E ε4 allele carriers. Most events (479 of 488 [98.2%]) among those with ARIA-E resolved radiographically; 404 of 488 (82.8%) resolved within 16 weeks. In the placebo group, 29 of 1076 participants (2.7%) had ARIA-E (apolipoprotein E ε4 carriers: 16 of 742 [2.2%]; noncarriers, 13 of 334 [3.9%]). ARIA-microhemorrhage and ARIA-superficial siderosis occurred in 197 participants (19.1%) and 151 participants (14.7%), respectively.
Conclusions and relevance: In this integrated safety data set from EMERGE and ENGAGE, the most common adverse event in the 10-mg/kg group was ARIA-E, which occurred in 362 of the 1029 patients (35.2%) in the 10-mg/kg group with at least 1 postbaseline MRI scan, with 94 patients (26.0%) experiencing associated symptoms. The most common associated symptom was headache.
Trial registrations: ClinicalTrials.gov Identifiers: NCT02484547, NCT02477800.
Conflict of interest statement
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

