Olefination via Cu-Mediated Dehydroacylation of Unstrained Ketones
- PMID: 34807585
- PMCID: PMC9116732
- DOI: 10.1021/jacs.1c09587
Olefination via Cu-Mediated Dehydroacylation of Unstrained Ketones
Abstract
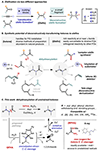
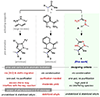
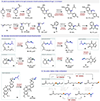
The dehydroacylation of ketones to olefins is realized under mild conditions, which exhibits a unique reaction pathway involving aromatization-driven C-C cleavage to remove the acyl moiety, followed by Cu-mediated oxidative elimination to form an alkene between the α and β carbons. The newly adopted N'-methylpicolinohydrazonamide (MPHA) reagent is key to enable efficient cleavage of ketone C-C bonds at room temperature. Diverse alkyl- and aryl-substituted olefins, dienes, and special alkenes are generated with broad functional group tolerance. Strategic applications of this method are also demonstrated.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- March J. March’s advanced organic chemistry: reactions, mechanisms, and structure. eighth. Smith MB editor. John Wiley & Sons, Inc., NJ. 2020, p 891–1085.
-
- Takeda T Modern Carbonyl Olefination: Methods and Applications; Wiley–VCH: Weinheim, Germany. 2006, p 1–338.
-
-
For selected reviews:
- Suess AM.; Lalic G. Copper-Catalyzed Hydrofunctionalization of Alkynes. Synlett 2016, 27, 1165–1174
- Zheng Y; Zi W. Transition-metal catalyzed Enantioselective Hydrofunctionalization of Alkynes. Tetrahedron Lett. 2018, 59, 2205–2213.
- Ananikov VP; Beletskaya IP; Tanaka M Hydrofunctionalization; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013, p 1–19.
-
-
-
For selected reviews, see:
- Flynn AB; Ogilvie WW Stereocontrolled Synthesis of Tetrasubstituted Olefins. Chem. Rev 2007, 107, 4698–4745; - PubMed
- Knowles JP; Whiting A The Heck–Mizoroki Cross-coupling Reaction: a Mechanistic Perspective. Org. Biomol. Chem 2007, 5, 31–44 - PubMed
- Larionov E; Li H; Mazet C Well-Defined Transition Metal Hydrides in Catalytic Isomerizations. Chem. Comm 2014, 50, 9816–9826; - PubMed
- Massad I; Marek I Alkene Isomerization through Allylmetals as a Strategic Tool in Stereoselective Synthesis. ACS Catal. 2020, 10, 5793–5804;
- Zhang J; Lu X; Shen C; Xu L; Ding L; Zhong G Recent advances in Chelation-Assisted Site-and Stereoselective Alkenyl C–H Functionalization. Chem. Soc. Rev 2021, 50, 3263–3314. - PubMed
-
-
- Bhawal BN; Morandi B Catalytic Transfer Functionalization through Shuttle Catalysis. ACS Catal. 2016, 6, 7528–7535;
- Morcillo SP Radical-Promoted C–C Bond Cleavage: A Deconstructive Approach for Selective Functionalization. Angew. Chem., Int. Ed 2019, 58, 14044–14054. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

