Crystal Structures of Two Titanium Phosphate-Based Proton Conductors: Ab Initio Structure Solution and Materials Properties
- PMID: 34807595
- PMCID: PMC8826274
- DOI: 10.1021/acs.inorgchem.1c02613
Crystal Structures of Two Titanium Phosphate-Based Proton Conductors: Ab Initio Structure Solution and Materials Properties
Abstract
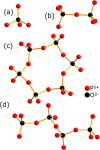
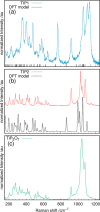
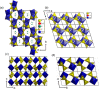
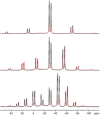
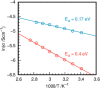
Transition-metal phosphates show a wide range of chemical compositions, variations of the valence states, and crystal structures. They are commercially used as solid-state catalysts, cathode materials in rechargeable batteries, or potential candidates for proton-exchange membranes in fuel cells. Here, we report on the successful ab initio structure determination of two novel titanium pyrophosphates, Ti(III)p and Ti(IV)p, from powder X-ray diffraction (PXRD) data. The low-symmetry space groups P21/c for Ti(III)p and P1̅ for Ti(IV)p required the combination of spectroscopic and diffraction techniques for structure determination. In Ti(III)p, trivalent titanium ions occupy the center of TiO6 polyhedra, coordinated by five pyrophosphate groups, one of them as a bidentate ligand. This secondary coordination causes the formation of one-dimensional six-membered ring channels with a diameter dmax of 3.93(2) Å, which is stabilized by NH4+ ions. Annealing Ti(III)p in inert atmospheres results in the formation of a new compound, denoted as Ti(IV)p. The structure of this compound shows a similar three-dimensional framework consisting of [PO4]3- tetrahedra and TiIV+O6 octahedra and an empty one-dimensional channel with a diameter dmax of 5.07(1) Å. The in situ PXRD of the transformation of Ti(III)p to Ti(IV)p reveals a two-step mechanism, i.e., the decomposition of NH4+ ions in a first step and subsequent structure relaxation. The specific proton conductivity and activation energy of the proton migration of Ti(III)p, governed by the Grotthus mechanism, belong to the highest and lowest, respectively, ever reported for this class of materials, which reveals its potential application in electrochemical devices like fuel cells and water electrolyzers in the intermediate temperature range.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Properties and Applications of Metal Phosphates and Pyrophosphates as Proton Conductors.Materials (Basel). 2022 Feb 9;15(4):1292. doi: 10.3390/ma15041292. Materials (Basel). 2022. PMID: 35207833 Free PMC article. Review.
-
Kinetic products in coordination networks: ab initio X-ray powder diffraction analysis.Acc Chem Res. 2013 Feb 19;46(2):493-505. doi: 10.1021/ar300212v. Epub 2012 Dec 19. Acc Chem Res. 2013. PMID: 23252592
-
Preparation and crystal structure of K2Ce(PO4)2: a new complex phosphate of Ce(iv) having structure with one-dimensional channels.Dalton Trans. 2016 Jan 21;45(3):980-91. doi: 10.1039/c5dt03288a. Dalton Trans. 2016. PMID: 26647831
-
Insight into ramsdellite LI(2)Ti(3)O(7) and its proton-exchange derivative.Inorg Chem. 2009 Aug 17;48(16):7659-66. doi: 10.1021/ic900398j. Inorg Chem. 2009. PMID: 19591438
-
Ca2[Ti(HPO4)2(PO4)]·H2O, Ca[Ti2(H2O)(HPO3)4]·H2O, and Ti(H2PO2)3: Solid-State Oxidation via Proton-Coupled Electron Transfer.Inorg Chem. 2022 Jan 24;61(3):1327-1334. doi: 10.1021/acs.inorgchem.1c02685. Epub 2022 Jan 7. Inorg Chem. 2022. PMID: 34994560
Cited by
-
Properties and Applications of Metal Phosphates and Pyrophosphates as Proton Conductors.Materials (Basel). 2022 Feb 9;15(4):1292. doi: 10.3390/ma15041292. Materials (Basel). 2022. PMID: 35207833 Free PMC article. Review.
-
Green steel from red mud through climate-neutral hydrogen plasma reduction.Nature. 2024 Jan;625(7996):703-709. doi: 10.1038/s41586-023-06901-z. Epub 2024 Jan 24. Nature. 2024. PMID: 38267679 Free PMC article.
-
Investigating the Reductive Phosphatization Reaction Pathway in the Synthesis of Transition Metal Phosphates: A Case Study on Titanium Phosphates.Inorg Chem. 2025 Feb 10;64(5):2425-2432. doi: 10.1021/acs.inorgchem.4c04776. Epub 2025 Jan 24. Inorg Chem. 2025. PMID: 39854175 Free PMC article.
References
-
- Hutchings G. J. Vanadium phosphate: a new look at the active components of catalysts for the oxidation of butane to maleic anhydride. J. Mater. Chem. 2004, 14, 3385–3395. 10.1039/b404610m. - DOI
-
- Delmas C.; Nadiri A.; Soubeyroux J. L. The nasicon-type titanium phosphates Ati2(PO4)3 (A = Li, Na) as electrode materials. Solid State Ionics 1988, 28–30, 419–423. 10.1016/S0167-2738(88)80075-4. - DOI
-
- Deniard P.; Dulac A. M.; Rocquefelte X.; Grigorova V.; Lebacq O.; Pasturel A.; Jobic S. High potential positive materials for lithium-ion batteries: transition metal phosphates. J. Phys. Chem. Solids 2004, 65 (2), 229–233. 10.1016/j.jpcs.2003.10.019. - DOI
-
- Hutchings G. J.; Kiely C. J.; Sananes-Schulz M. T.; Burrows A.; Volta J. C. Comments on the nature of the active site of vanadium phosphate catalysts for butane oxidation. Catal. Today 1998, 40 (2), 273–286. 10.1016/S0920-5861(98)00015-7. - DOI
-
- Jin Y.; Shen Y.; Hibino T. Proton conduction in metal pyrophosphates (MP2O7) at intermediate temperatures. J. Mater. Chem. 2010, 20, 6214–6217. 10.1039/b924188d. - DOI
LinkOut - more resources
Full Text Sources

