Identification of FtpA, a Dps-Like Protein Involved in Anti-Oxidative Stress and Virulence in Actinobacillus pleuropneumoniae
- PMID: 34807725
- PMCID: PMC8846326
- DOI: 10.1128/JB.00326-21
Identification of FtpA, a Dps-Like Protein Involved in Anti-Oxidative Stress and Virulence in Actinobacillus pleuropneumoniae
Abstract
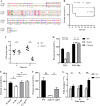
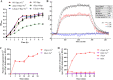
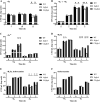
Bacteria have evolved a variety of enzymes to eliminate endogenous or host-derived oxidative stress factors. The Dps protein, first identified in Escherichia coli, contains a ferroxidase center, and protects bacteria from reactive oxygen species damage. Little is known of the role of Dps-like proteins in bacterial pathogenesis. Actinobacillus pleuropneumoniae causes pleuropneumonia, a respiratory disease of swine. The A. pleuropneumoniae ftpA gene is upregulated during shifts to anaerobiosis, in biofilms and, as found in this study, in the presence of H2O2. An A. pleuropneumoniae ftpA deletion mutant (ΔftpA) had increased H2O2 sensitivity, decreased intracellular viability in macrophages, and decreased virulence in a mouse infection model. Expression of ftpA in an E. coli dps mutant restored wild-type H2O2 resistance. FtpA possesses a conserved ferritin domain containing a ferroxidase site. Recombinant rFtpA bound and oxidized Fe2+ reversibly. Under aerobic conditions, the viability of an ΔftpA mutant was reduced compared with the wild-type strain after extended culture, upon transition from anaerobic to aerobic conditions, and upon supplementation with Fenton reaction substrates. Under anaerobic conditions, the addition of H2O2 resulted in a more severe growth defect of ΔftpA than it did under aerobic conditions. Therefore, by oxidizing and mineralizing Fe2+, FtpA alleviates the oxidative damage mediated by intracellular Fenton reactions. Furthermore, by mutational analysis, two residues were confirmed to be critical for Fe2+ binding and oxidization, as well as for A. pleuropneumoniae H2O2 resistance. Taken together, the results of this study demonstrate that A. pleuropneumoniae FtpA is a Dps-like protein, playing critical roles in oxidative stress resistance and virulence. IMPORTANCE As a ferroxidase, Dps of Escherichia coli can protect bacteria from reactive oxygen species damage, but its role in bacterial pathogenesis has received little attention. In this study, FtpA of the swine respiratory pathogen A. pleuropneumoniae was identified as a new Dps-like protein. It facilitated A. pleuropneumoniae resistance to H2O2, survival in macrophages, and infection in vivo. FtpA could bind and oxidize Fe2+ through two important residues in its ferroxidase site and protected the bacteria from oxidative damage mediated by the intracellular Fenton reaction. These findings provide new insights into the role of the FtpA-based antioxidant system in the pathogenesis of A. pleuropneumoniae, and the conserved Fe2+ binding ligands in Dps/FtpA provide novel drug target candidates for disease prevention.
Keywords: Actinobacillus pleuropneumoniae; Dps; Fenton reaction; FtpA; ferroxidase; oxidative stress; virulence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
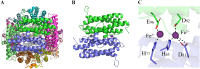

Similar articles
-
The antioxidant protein PntA coordinates with OmpW to resist oxidant stress in Actinobacillus pleuropneumoniae.Vet Microbiol. 2025 May;304:110500. doi: 10.1016/j.vetmic.2025.110500. Epub 2025 Mar 29. Vet Microbiol. 2025. PMID: 40174305
-
A requirement of TolC1 for effective survival, colonization and pathogenicity of Actinobacillus pleuropneumoniae.Microb Pathog. 2019 Sep;134:103596. doi: 10.1016/j.micpath.2019.103596. Epub 2019 Jun 15. Microb Pathog. 2019. PMID: 31212036
-
Identification and characterization of a novel stress-responsive outer membrane protein Lip40 from Actinobacillus pleuropneumoniae.BMC Biotechnol. 2015 Nov 25;15:106. doi: 10.1186/s12896-015-0199-8. BMC Biotechnol. 2015. PMID: 26608465 Free PMC article.
-
Virulence factors of the swine pathogen Actinobacillus pleuropneumoniae.Microbiologia. 1996 Jun;12(2):171-84. Microbiologia. 1996. PMID: 8767702 Review.
-
Surface polysaccharides and iron-uptake systems of Actinobacillus pleuropneumoniae.Can J Vet Res. 2004 Apr;68(2):81-5. Can J Vet Res. 2004. PMID: 15188950 Free PMC article. Review.
Cited by
-
Naringin attenuates Actinobacillus pleuropneumoniae-induced acute lung injury via MAPK/NF-κB and Keap1/Nrf2/HO-1 pathway.BMC Vet Res. 2024 May 17;20(1):204. doi: 10.1186/s12917-024-04055-2. BMC Vet Res. 2024. PMID: 38755662 Free PMC article.
-
Functional flexibility of a type III polyketide synthase in Mycobacterium marinum.iScience. 2025 Jul 16;28(8):113129. doi: 10.1016/j.isci.2025.113129. eCollection 2025 Aug 15. iScience. 2025. PMID: 40777054 Free PMC article.
-
The association of gut microbiota, immunocyte dynamics, and protein-protein ratios with tuberculosis susceptibility: a Mendelian randomization analysis.Sci Rep. 2025 Jan 23;15(1):2931. doi: 10.1038/s41598-025-87160-y. Sci Rep. 2025. PMID: 39849060 Free PMC article.
-
Naringin's Alleviation of the Inflammatory Response Caused by Actinobacillus pleuropneumoniae by Downregulating the NF-κB/NLRP3 Signalling Pathway.Int J Mol Sci. 2024 Jan 14;25(2):1027. doi: 10.3390/ijms25021027. Int J Mol Sci. 2024. PMID: 38256101 Free PMC article.
-
Actinobacillus pleuropneumoniae FliY and YdjN are involved in cysteine/cystine utilization, oxidative resistance, and biofilm formation but are not determinants of virulence.Front Microbiol. 2023 May 12;14:1169774. doi: 10.3389/fmicb.2023.1169774. eCollection 2023. Front Microbiol. 2023. PMID: 37250053 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

