Organic Acids and Their Salts Potentiate the Activity of Selected Antibiotics against Pseudomonas aeruginosa Biofilms Grown in a Synthetic Cystic Fibrosis Sputum Medium
- PMID: 34807756
- PMCID: PMC8765270
- DOI: 10.1128/AAC.01875-21
Organic Acids and Their Salts Potentiate the Activity of Selected Antibiotics against Pseudomonas aeruginosa Biofilms Grown in a Synthetic Cystic Fibrosis Sputum Medium
Abstract
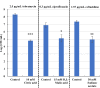
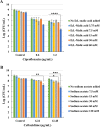
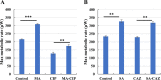
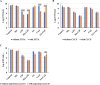
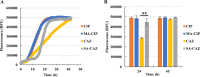
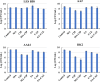
The failure of antibiotic therapy in respiratory tract infections in cystic fibrosis is partly due to the high tolerance observed in Pseudomonas aeruginosa biofilms. This tolerance is mediated by changes in bacterial metabolism linked to growth in biofilms, opening up potential avenues for novel treatment approaches based on modulating metabolism. The goal of the present study was to identify carbon sources that increase the inhibiting and/or eradicating activity of tobramycin, ciprofloxacin, and ceftazidime against P. aeruginosa PAO1 biofilms grown in a synthetic cystic fibrosis sputum medium (SCFM2) and to elucidate their mode of action. After screening 69 carbon sources, several combinations of antibiotics + carbon sources that showed markedly higher anti-biofilm activity than antibiotics alone were identified. d,l-malic acid and sodium acetate could potentiate both biofilm inhibiting and eradicating activity of ciprofloxacin and ceftazidime, respectively, while citric acid could only potentiate biofilm inhibitory activity of tobramycin. The mechanisms underlying the increased biofilm eradicating activity of combinations ciprofloxacin/d,l-malic acid and ceftazidime/sodium acetate are similar but not identical. Potentiation of ceftazidime activity by sodium acetate was linked to increased metabolic activity, a functional TCA cycle, increased ROS production, and high intracellular pH, whereas the latter was not required for d,l-malic acid potentiation of ciprofloxacin. Finally, our results indicate that the potentiation of antibiotic activity by carbon sources is strain dependent.
Keywords: Pseudomonas aeruginosa; biofilms; cystic fibrosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Activity of Antibiotics against Pseudomonas aeruginosa in an In Vitro Model of Biofilms in the Context of Cystic Fibrosis: Influence of the Culture Medium.Antimicrob Agents Chemother. 2020 Mar 24;64(4):e02204-19. doi: 10.1128/AAC.02204-19. Print 2020 Mar 24. Antimicrob Agents Chemother. 2020. PMID: 32015047 Free PMC article.
-
Repurposing DNase I and alginate lyase to degrade the biofilm matrix of dual-species biofilms of Staphylococcus aureus and Pseudomonas aeruginosa grown in artificial sputum medium: In-vitro assessment of their activity in combination with broad-spectrum antibiotics.J Cyst Fibros. 2024 Nov;23(6):1146-1152. doi: 10.1016/j.jcf.2024.02.012. Epub 2024 Feb 24. J Cyst Fibros. 2024. PMID: 38402083
-
Bacteriophages as potential antibiotic potentiators in cystic fibrosis: A new model to study the combination of antibiotics with a bacteriophage cocktail targeting dual species biofilms of Staphylococcus aureus and Pseudomonas aeruginosa.Int J Antimicrob Agents. 2024 Sep;64(3):107276. doi: 10.1016/j.ijantimicag.2024.107276. Epub 2024 Jul 14. Int J Antimicrob Agents. 2024. PMID: 39009289
-
Standard versus biofilm antimicrobial susceptibility testing to guide antibiotic therapy in cystic fibrosis.Cochrane Database Syst Rev. 2020 Jun 10;6(6):CD009528. doi: 10.1002/14651858.CD009528.pub5. Cochrane Database Syst Rev. 2020. PMID: 32520436 Free PMC article.
-
Inhaled tobramycin (TOBI): a review of its use in the management of Pseudomonas aeruginosa infections in patients with cystic fibrosis.Drugs. 2003;63(22):2501-20. doi: 10.2165/00003495-200363220-00015. Drugs. 2003. PMID: 14609360 Review.
Cited by
-
Antibiofilm Activities of Borneol-Citral-Loaded Pickering Emulsions against Pseudomonas aeruginosa and Staphylococcus aureus in Physiologically Relevant Chronic Infection Models.Microbiol Spectr. 2022 Oct 26;10(5):e0169622. doi: 10.1128/spectrum.01696-22. Epub 2022 Oct 4. Microbiol Spectr. 2022. PMID: 36194139 Free PMC article.
-
The biofilm community resurfaces: new findings and post-pandemic progress.J Bacteriol. 2023 Oct 26;205(10):e0016623. doi: 10.1128/jb.00166-23. Epub 2023 Sep 27. J Bacteriol. 2023. PMID: 37756166 Free PMC article. Review.
-
Mechanistic Insights into Succinic Acid as an Adjuvant for Ciprofloxacin in Treating Pseudomonas aeruginosa Growing Within Cystic Fibrosis Airway Mucus.Microorganisms. 2024 Dec 9;12(12):2538. doi: 10.3390/microorganisms12122538. Microorganisms. 2024. PMID: 39770741 Free PMC article.
-
Adaptation of Pseudomonas aeruginosa biofilms to tobramycin and the quorum sensing inhibitor C-30 during experimental evolution requires multiple genotypic and phenotypic changes.Microbiology (Reading). 2023 Jan;169(1):001278. doi: 10.1099/mic.0.001278. Microbiology (Reading). 2023. PMID: 36748633 Free PMC article.
-
Mechanisms of antibiotic resistance in Pseudomonas aeruginosa biofilms.Biofilm. 2023 May 2;5:100129. doi: 10.1016/j.bioflm.2023.100129. eCollection 2023 Dec. Biofilm. 2023. PMID: 37205903 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

