Hybrid dedicated and distributed coding in PMd/M1 provides separation and interaction of bilateral arm signals
- PMID: 34807905
- PMCID: PMC8648118
- DOI: 10.1371/journal.pcbi.1009615
Hybrid dedicated and distributed coding in PMd/M1 provides separation and interaction of bilateral arm signals
Abstract
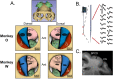
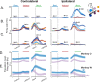
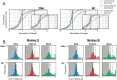
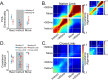
Pronounced activity is observed in both hemispheres of the motor cortex during preparation and execution of unimanual movements. The organizational principles of bi-hemispheric signals and the functions they serve throughout motor planning remain unclear. Using an instructed-delay reaching task in monkeys, we identified two components in population responses spanning PMd and M1. A "dedicated" component, which segregated activity at the level of individual units, emerged in PMd during preparation. It was most prominent following movement when M1 became strongly engaged, and principally involved the contralateral hemisphere. In contrast to recent reports, these dedicated signals solely accounted for divergence of arm-specific neural subspaces. The other "distributed" component mixed signals for each arm within units, and the subspace containing it did not discriminate between arms at any stage. The statistics of the population response suggest two functional aspects of the cortical network: one that spans both hemispheres for supporting preparatory and ongoing processes, and another that is predominantly housed in the contralateral hemisphere and specifies unilateral output.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

