Mammarenaviruses of Rodents, South Africa and Zimbabwe
- PMID: 34808083
- PMCID: PMC8632164
- DOI: 10.3201/eid2712.211088
Mammarenaviruses of Rodents, South Africa and Zimbabwe
Abstract
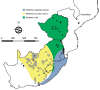
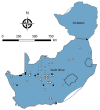
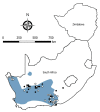
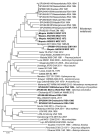
We conducted a survey for group-specific indirect immunofluorescence antibody to mammarenaviruses by using Lassa fever and Mopeia virus antigens on serum specimens of 5,363 rodents of 33 species collected in South Africa and Zimbabwe during 1964-1994. Rodents were collected for unrelated purposes or for this study and stored at -70°C. We found antibody to be widely distributed in the 2 countries; antibody was detected in serum specimens of 1.2%-31.8% of 14 species of myomorph rodents, whereas 19 mammarenavirus isolates were obtained from serum specimens and viscera of 4 seropositive species. Phylogenetic analysis on the basis of partial nucleoprotein sequences indicates that 14 isolates from Mastomys natalensis, the Natal multimammate mouse, were Mopeia virus, whereas Merino Walk virus was characterized as a novel virus in a separate study. The remaining 4 isolates from 3 rodent species potentially constitute novel viruses pending full characterization.
Keywords: Mastomys natalensis; Mopeia virus; Natal multimammate mouse; South Africa; Zimbabwe; mammarenavirus; rodents; viruses.
Figures


References
-
- Swanepoel R. Viral haemorrhagic fevers in South Africa: history and national strategy. S Afr J Sci. 1987;83:80–8.
MeSH terms
LinkOut - more resources
Full Text Sources

