Improving Compliance With Revised Newborn Hepatitis B Vaccination Policy
- PMID: 34808667
- PMCID: PMC9843611
- DOI: 10.1542/hpeds.2021-005969
Improving Compliance With Revised Newborn Hepatitis B Vaccination Policy
Abstract
Background: In September 2017, the American Academy of Pediatrics issued guidance recommending hepatitis B vaccine be administered to well newborns with birth weight ≥2000 g within 24 hours after birth. At that time, ∼85% of well newborns were vaccinated before discharge at our center; however, only 35% were vaccinated within 24 hours after birth. Our aim was to vaccinate 70% of eligible newborns within 24 hours after birth by June 2018 while maintaining the overall rate of vaccination.
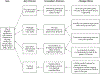
Methods: A multidisciplinary improvement team analyzed existing vaccine administration processes in the well-newborn nursery. From October 2017 to January 2018, changes were made to activation of vaccine orders and to obtaining and documenting the consent processes. Vaccine administration was bundled with routine care given ≤24 hours after birth, and parent scripting was changed from offering vaccine as an option to stating it as a recommendation. From November 2016 to June 2019, we determined the overall rate and timing of vaccination using statistical process control methods.
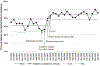
Results: Among 10 887 eligible infants, the proportion administered hepatitis B vaccine ≤24 hours after birth increased from 35.5% to 78.8% after process changes with special-cause variation on process control charts. Proportion of infants receiving vaccine any time before discharge also increased from 86.5% to 92.3%.
Conclusions: Specific process changes allowed our birth center to comply with the recommended timing for hepatitis B vaccination of ≤24 hours after birth among eligible newborns.
Copyright © 2021 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Figures
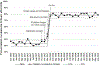
References
-
- Immunization Action Coalition. Hepatitis B: What hospitals need to do to protect newborns. In: Hepatitis B: What Hospitals Need to do to Protect Newborns. Saint Paul, Minnesota: Immunization Action Coalition; 2016:10–21.
-
- CDC, National Notifiable Diseases Surveillance System. Viral Hepatitis Surveillance. Available at: https://www.cdc.gov/hepatitis/statistics/2018surveillance/HepB.htm. Accessed 1/14/, 2021.
-
- Schillie S, Walker T, Veselsky S, et al. Outcomes of Infants Born to Women Infected With Hepatitis B. Pediatrics (Evanston). 2015;135:e1141-e1147. - PubMed
-
- AAP Committee on Infectious Diseases and AAP Committee on Fetus and Newborn. Elimination of perinatal hepatitis B: providing the first vaccine dose within 24 hours of birth. Pediatrics (Evanston). 2017;140:e20171870. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

