SOS-Independent Pyocin Production in P. aeruginosa Is Induced by XerC Recombinase Deficiency
- PMID: 34809462
- PMCID: PMC8609362
- DOI: 10.1128/mBio.02893-21
SOS-Independent Pyocin Production in P. aeruginosa Is Induced by XerC Recombinase Deficiency
Abstract
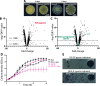
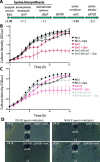
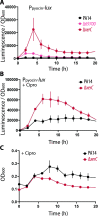
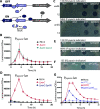
Pyocins are phage tail-like protein complexes that can be used by Pseudomonas aeruginosa to enact intraspecies competition by killing competing strains. The pyocin gene cluster also encodes holin and lysin enzymes that lyse producer cells to release the pyocins. The best-known inducers of pyocin production under laboratory conditions are DNA-damaging agents, including fluoroquinolone antibiotics, that activate the SOS response. Here, we report the discovery of an alternate, RecA-independent pathway of strong pyocin induction that is active in cells deficient for the tyrosine recombinase XerC. When ΔxerC cells were examined at the single-cell level, only a fraction of the cell population strongly expressed pyocins before explosively lysing, suggesting a that a built-in heterogenous response system protects the cell population from widespread lysis. Disabling the holin and lysin enzymes or deleting the entire pyocin gene cluster blocked explosive lysis and delayed but did not prevent the death of pyocin-producing cells, suggesting that ΔxerC cells activate other lysis pathways. Mutating XerC to abolish its recombinase activity induced pyocin expression to a lesser extent than the full deletion, suggesting that XerC has multiple functions with respect to pyocin activation. Our studies uncover a new pathway for pyocin production and highlight its response across a genetically identical population. Moreover, our finding that ΔxerC populations are hypersensitive to fluoroquinolones raises the intriguing possibility that XerC inhibition may potentiate the activity of these antibiotics against P. aeruginosa infections. IMPORTANCE Pseudomonas aeruginosa is a versatile and ubiquitous bacterium that frequently infects humans as an opportunistic pathogen. P. aeruginosa competes with other strains within the species by producing killing complexes termed pyocins, which are only known to be induced by cells experiencing DNA damage and the subsequent SOS response. Here, we discovered that strains lacking a recombinase enzyme called XerC strongly produce pyocins independently of the SOS response. We also show that these strains are hypersensitive to commonly used fluoroquinolone antibiotic treatment and that fluoroquinolones further stimulate pyocin production. Thus, XerC is an attractive target for future therapies that simultaneously sensitize P. aeruginosa to antibiotics and stimulate the production of bactericidal pyocins.
Keywords: Pseudomonas aeruginosa; competition; heterogeneity; pyocins; recombinase.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
