Hydroxypropylmethylcellulose as a film and hydrogel carrier for ACP nanoprecursors to deliver biomimetic mineralization
- PMID: 34809623
- PMCID: PMC8607665
- DOI: 10.1186/s12951-021-01133-7
Hydroxypropylmethylcellulose as a film and hydrogel carrier for ACP nanoprecursors to deliver biomimetic mineralization
Erratum in
-
Correction to: Hydroxypropylmethylcellulose as a film and hydrogel carrier for ACP nanoprecursors to deliver biomimetic mineralization.J Nanobiotechnology. 2021 Dec 18;19(1):424. doi: 10.1186/s12951-021-01170-2. J Nanobiotechnology. 2021. PMID: 34922535 Free PMC article. No abstract available.
Abstract
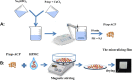
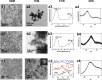
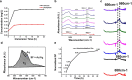
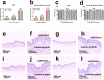
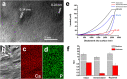
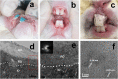
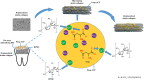
Demineralization of hard tooth tissues leads to dental caries, which cause health problems and economic burdens throughout the world. A biomimetic mineralization strategy is expected to reverse early dental caries. Commercially available anti-carious mineralizing products lead to inconclusive clinical results because they cannot continuously replenish the required calcium and phosphate resources. Herein, we prepared a mineralizing film consisting of hydroxypropylmethylcellulose (HPMC) and polyaspartic acid-stabilized amorphous calcium phosphate (PAsp-ACP) nanoparticles. HPMC which contains multiple hydroxyl groups is a film-forming material that can be desiccated to form a dry film. In a moist environment, this film gradually changes into a gel. HPMC was used as the carrier of PAsp-ACP nanoparticles to deliver biomimetic mineralization. Our results indicated that the hydroxyl and methoxyl groups of HPMC could assist the stability of PAsp-ACP nanoparticles and maintain their biomimetic mineralization activity. The results further demonstrated that the bioinspired mineralizing film induced the early mineralization of demineralized dentin after 24 h with increasing mineralization of the whole demineralized dentin (3-4 µm) after 72-96 h. Furthermore, these results were achieved without any cytotoxicity or mucosa irritation. Therefore, this mineralizing film shows promise for use in preventive dentistry due to its efficient mineralization capability.
Keywords: Biomimetic mineralization; Collagen; Dental caries; Dentin; Film; Hydroxypropylmethylcellulose.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Peres MA, Macpherson LMD, Weyant RJ, Daly B, Venturelli R, Mathur MR, et al. Oral diseases: a global public health challenge. Lancet. 2019;394(10194):249–260. - PubMed
-
- Bernabé E, Marcenes W. Can minimal intervention dentistry help in tackling the global burden of untreated dental caries? Br Dent J. 2020;229(7):487–491. - PubMed
-
- Merchan MT, Ismail AI. Measurement and distribution of dental caries. In: Burt and Eklund’s dentistry, dental practice, and the community. Seventh Edition. 2021. p. 154–70. 10.1016/b978-0-323-55484-8.00014-9.
-
- Vanaei S, Parizi MS, Vanaei S, Salemizadehparizi F, Vanaei HR. An overview on materials and techniques in 3D bioprinting toward biomedical application. Eng Regener. 2021;2:1–18.
-
- Feng X, Hou X, Cui C, Sun S, Sadik S, Wu S, et al. Mechanical and antibacterial properties of tannic acid-encapsulated carboxymethyl chitosan/polyvinyl alcohol hydrogels. Eng Regener. 2021;2:57–62.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

