STAT proteins: a kaleidoscope of canonical and non-canonical functions in immunity and cancer
- PMID: 34809691
- PMCID: PMC8607625
- DOI: 10.1186/s13045-021-01214-y
STAT proteins: a kaleidoscope of canonical and non-canonical functions in immunity and cancer
Abstract
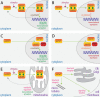
STAT proteins represent an important family of evolutionarily conserved transcription factors that play key roles in diverse biological processes, notably including blood and immune cell development and function. Classically, STAT proteins have been viewed as inducible activators of transcription that mediate cellular responses to extracellular signals, particularly cytokines. In this 'canonical' paradigm, latent STAT proteins become tyrosine phosphorylated following receptor activation, typically via downstream JAK proteins, facilitating their dimerization and translocation into the nucleus where they bind to specific sequences in the regulatory region of target genes to activate transcription. However, growing evidence has challenged this paradigm and identified alternate 'non-canonical' functions, such as transcriptional repression and roles outside the nucleus, with both phosphorylated and unphosphorylated STATs involved. This review provides a revised framework for understanding the diverse kaleidoscope of STAT protein functional modalities. It further discusses the implications of this framework for our understanding of STAT proteins in normal blood and immune cell biology and diseases such as cancer, and also provides an evolutionary context to place the origins of these alternative functional modalities.
Keywords: Cancer; Cytokine; Immunity; JAK; STAT; Transcription factor.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
-
- Shuai K, Stark GR, Kerr IM, Darnell JE. A single phosphotyrosine residue of Stat91 required for gene activation by interferon-gamma. Science. 1993;261(5129):1744–1746. - PubMed
-
- Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. - PubMed
-
- Zhong Z, Wen Z, Darnell JE. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264(5155):95–98. - PubMed
-
- Ram PA, Park S-H, Choi HK, Waxman DJ. Growth hormone activation of Stat1, Stat3, and Stat5 in rat liver: differential kinetics of hormone desensitization and growth hormone stimulation of both tyrosine phosphorylation and serine/threonine phoshorylation. J Biol Chem. 1996;271(10):5929–5940. - PubMed
-
- Amiri F, Shaw S, Wang X, Tang J, Waller JL, Eaton DC, et al. Angiotensin II activation of the JAK/STAT pathway in mesangial cells is altered by high glucose. Kidney Int. 2002;61(5):1605–1616. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

