Mitochondrial regulation and white adipose tissue homeostasis
- PMID: 34810062
- PMCID: PMC8918005
- DOI: 10.1016/j.tcb.2021.10.008
Mitochondrial regulation and white adipose tissue homeostasis
Abstract
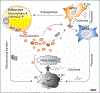
The important role of mitochondria in the regulation of white adipose tissue (WAT) remodeling and energy balance is increasingly appreciated. The remarkable heterogeneity of the adipose tissue stroma provides a cellular basis to enable adipose tissue plasticity in response to various metabolic stimuli. Regulating mitochondrial function at the cellular level in adipocytes, in adipose progenitor cells (APCs), and in adipose tissue macrophages (ATMs) has a profound impact on adipose homeostasis. Moreover, mitochondria facilitate the cell-to-cell communication within WAT, as well as the crosstalk with other organs, such as the liver, the heart, and the pancreas. A better understanding of mitochondrial regulation in the diverse adipose tissue cell types allows us to develop more specific and efficient approaches to improve adipose function and achieve improvements in overall metabolic health.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no conflicts of interest.
Figures


Similar articles
-
The Emerging Importance of Mitochondria in White Adipocytes: Neither Last nor Least.Endocrinol Metab (Seoul). 2023 Oct;38(5):493-503. doi: 10.3803/EnM.2023.1813. Epub 2023 Oct 10. Endocrinol Metab (Seoul). 2023. PMID: 37816498 Free PMC article.
-
Cellular and Intercellular Homeostasis in Adipose Tissue with Mitochondria-Specific Stress.Endocrinol Metab (Seoul). 2021 Feb;36(1):1-11. doi: 10.3803/EnM.2021.956. Epub 2021 Feb 24. Endocrinol Metab (Seoul). 2021. PMID: 33677920 Free PMC article.
-
Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome.Autophagy. 2019 Nov;15(11):1882-1898. doi: 10.1080/15548627.2019.1596482. Epub 2019 Apr 6. Autophagy. 2019. PMID: 30898010 Free PMC article.
-
White adipose tissue mitochondrial metabolism in health and in obesity.Obes Rev. 2020 Feb;21(2):e12958. doi: 10.1111/obr.12958. Epub 2019 Nov 27. Obes Rev. 2020. PMID: 31777187 Review.
-
White adipose tissue mitochondrial bioenergetics in metabolic diseases.Rev Endocr Metab Disord. 2023 Dec;24(6):1121-1133. doi: 10.1007/s11154-023-09827-z. Epub 2023 Aug 10. Rev Endocr Metab Disord. 2023. PMID: 37558853 Review.
Cited by
-
Obesity-Related Adipose Tissue Remodeling in the Light of Extracellular Mitochondria Transfer.Int J Mol Sci. 2022 Jan 6;23(2):632. doi: 10.3390/ijms23020632. Int J Mol Sci. 2022. PMID: 35054817 Free PMC article. Review.
-
The Effect of Sleep Disruption on Cardiometabolic Health.Life (Basel). 2025 Jan 7;15(1):60. doi: 10.3390/life15010060. Life (Basel). 2025. PMID: 39860000 Free PMC article. Review.
-
Untargeted Metabolite Profiling Reveals Acute Toxicity of Pentosidine on Adipose Tissue of Rats.Metabolites. 2024 Oct 9;14(10):539. doi: 10.3390/metabo14100539. Metabolites. 2024. PMID: 39452920 Free PMC article.
-
Cryptotanshinone promotes brown fat activity by AMPK activation to inhibit obesity.Nutr Res Pract. 2024 Aug;18(4):479-497. doi: 10.4162/nrp.2024.18.4.479. Epub 2024 May 23. Nutr Res Pract. 2024. PMID: 39109201 Free PMC article.
-
Recent advances in the crosstalk between adipose, muscle and bone tissues in fish.Front Endocrinol (Lausanne). 2023 Mar 14;14:1155202. doi: 10.3389/fendo.2023.1155202. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36998471 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

