NAADP-binding proteins find their identity
- PMID: 34810081
- PMCID: PMC8840967
- DOI: 10.1016/j.tibs.2021.10.008
NAADP-binding proteins find their identity
Abstract
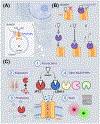
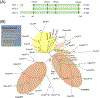
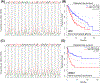
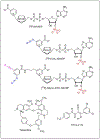
Nicotinic acid adenine dinucleotide phosphate (NAADP) is a second messenger that releases Ca2+ from endosomes and lysosomes by activating ion channels called two-pore channels (TPCs). However, no NAADP-binding site has been identified on TPCs. Rather, NAADP activates TPCs indirectly by engaging NAADP-binding proteins (NAADP-BPs) that form part of the TPC complex. After a decade of searching, two different NAADP-BPs were recently identified: Jupiter microtubule associated homolog 2 (JPT2) and like-Sm protein 12 (LSM12). These discoveries bridge the gap between NAADP generation and NAADP activation of TPCs, providing new opportunity to understand and manipulate the NAADP-signaling pathway. The unmasking of these NAADP-BPs will catalyze future studies to define the molecular choreography of NAADP action.
Keywords: calcium signaling; cancer; endosomes; lysosomes; photoaffinity probes.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests No interests are declared.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM088790/GM/NIGMS NIH HHS/United States
- R15 GM131329/GM/NIGMS NIH HHS/United States
- T32 HL007895/HL/NHLBI NIH HHS/United States
- BB/N01524X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/T015853/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Miscellaneous

