Efficacy and safety of colchicine in COVID-19: a meta-analysis of randomised controlled trials
- PMID: 34810227
- PMCID: PMC8561824
- DOI: 10.1136/rmdopen-2021-001746
Efficacy and safety of colchicine in COVID-19: a meta-analysis of randomised controlled trials
Abstract
Background: Colchicine, an anti-inflammatory drug is prescribed nowadays for COVID-19. In this meta-analysis, we evaluated efficacy and safety of colchicine in patients with COVID-19.
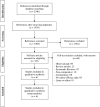
Methods: We searched databases for randomised controlled studies evaluating efficacy and/or safety of colchicine as compared with supportive care in patients with COVID-19. The efficacy outcomes were mortality, ventilatory support, intensive care unit (ICU) admission and length of hospital stay. The safety outcomes were adverse events, serious adverse events and diarrhoea. A meta-analytical summary was estimated using random effects model through Mantle-Hanzle method. An I2 test was used to assess heterogeneity. The Grades of Recommendation, Assessment, Development and Evaluation (GRADE) approach was used to assess quality of evidence for each outcome.
Results: Out of 69 full texts assessed, 6 studies (16148 patients with COVID-19) were included in meta-analysis. Patients receiving colchicine did not show significant reduction in mortality (risk difference, RD -0.00 (95% CI -0.01 to 0.01), I2=15%), ventilatory support (risk ratio, RR 0.67 (95% CI 0.38 to 1.21), I2=47%), ICU admission (RR 0.49 (95% CI 0.19 to 1.25), I2=34%), length of hospital stay (mean difference: -1.17 (95% CI -3.02 to 0.67), I2=77%) and serious adverse events (RD -0.01 (95% CI -0.02 to 0.00), I2=28%) than those who received supportive care only. Patients receiving colchicine had higher rates of adverse events (RR 1.58 (95% CI 1.07 to 2.33), I2=81%) and diarrhoea (RR 1.93 (95% CI 1.62 to 2.29), I2=0%) than supportive care treated patients. The GRADE quality of evidence was moderate for most outcomes.
Conclusion: The moderate quality evidence suggests no benefit of addition of colchicine to the standard care regimen in patients with COVID-19.
Keywords: COVID-19; anti-inflammatory agents; cytokines; non-steroidal.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures



Comment on
-
Beneficial effects of colchicine for moderate to severe COVID-19: a randomised, double-blinded, placebo-controlled clinical trial.RMD Open. 2021 Feb;7(1):e001455. doi: 10.1136/rmdopen-2020-001455. RMD Open. 2021. PMID: 33542047 Free PMC article. Clinical Trial.
-
Proactive anti-inflammatory therapy with colchicine in the treatment of advanced stages of new coronavirus infection. The first results of the COLORIT study.Kardiologiia. 2021 Mar 1;61(2):15-27. doi: 10.18087/cardio.2021.2.n1560. Kardiologiia. 2021. PMID: 33734043 Clinical Trial.
References
-
- World Health Organisation . Coronavirus disease (COVID-19) situation reports, 2021. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situatio...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
