Prospective validation of the 4C prognostic models for adults hospitalised with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol
- PMID: 34810237
- PMCID: PMC8610617
- DOI: 10.1136/thoraxjnl-2021-217629
Prospective validation of the 4C prognostic models for adults hospitalised with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol
Abstract
Purpose: To prospectively validate two risk scores to predict mortality (4C Mortality) and in-hospital deterioration (4C Deterioration) among adults hospitalised with COVID-19.
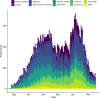
Methods: Prospective observational cohort study of adults (age ≥18 years) with confirmed or highly suspected COVID-19 recruited into the International Severe Acute Respiratory and emerging Infections Consortium (ISARIC) WHO Clinical Characterisation Protocol UK (CCP-UK) study in 306 hospitals across England, Scotland and Wales. Patients were recruited between 27 August 2020 and 17 February 2021, with at least 4 weeks follow-up before final data extraction. The main outcome measures were discrimination and calibration of models for in-hospital deterioration (defined as any requirement of ventilatory support or critical care, or death) and mortality, incorporating predefined subgroups.
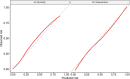
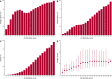
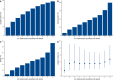
Results: 76 588 participants were included, of whom 27 352 (37.4%) deteriorated and 12 581 (17.4%) died. Both the 4C Mortality (0.78 (0.77 to 0.78)) and 4C Deterioration scores (pooled C-statistic 0.76 (95% CI 0.75 to 0.77)) demonstrated consistent discrimination across all nine National Health Service regions, with similar performance metrics to the original validation cohorts. Calibration remained stable (4C Mortality: pooled slope 1.09, pooled calibration-in-the-large 0.12; 4C Deterioration: 1.00, -0.04), with no need for temporal recalibration during the second UK pandemic wave of hospital admissions.
Conclusion: Both 4C risk stratification models demonstrate consistent performance to predict clinical deterioration and mortality in a large prospective second wave validation cohort of UK patients. Despite recent advances in the treatment and management of adults hospitalised with COVID-19, both scores can continue to inform clinical decision making.
Trial registration number: ISRCTN66726260.
Keywords: COVID-19.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: AD reports grants from Department of Health and Social Care, during the conduct of the study; grants from Wellcome Trust, outside the submitted work; CG reports grants from DHSC National Institute of Health Research UK, during the conduct of the study; PH reports grants from Wellcome Trust/Department for International Development/Bill and Melinda Gates Foundation, grants from NIHR, during the conduct of the study; JSN-V-T reports grants from Department of Health and Social Care, England, during the conduct of the study and is seconded to the Department of Health and Social Care, England (DHSC); MN is supported by a Wellcome Trust investigator award and the NIHR University College London Hospitals Biomedical Research Centre (BRC). PJO reports personal fees from consultancies and from European Respiratory Society; grants from MRC, MRC Global Challenge Research Fund, EU, NIHR BRC, MRC/GSK, Wellcome Trust, NIHR (HPRU in Respiratory Infection), and NIHR Senior Investigator outside the submitted work. His role as President of the British Society for Immunology was unpaid but travel and accommodation at some meetings was provided by the Society. JKB reports grants from Medical Research Council UK; MGS reports grants from DHSC National Institute of Health Research UK, grants from Medical Research Council UK, grants from Health Protection Research Unit in Emerging & Zoonotic Infections, University of Liverpool, during the conduct of the study; other from Integrum Scientific LLC, Greensboro, NC, USA, outside the submitted work. LT reports grants from Health Protection Research Unit in Emerging & Zoonotic Infections, University of Liverpool, during the conduct of the study and grants from the Wellcome Trust outside the submitted work. EH, HA, JD, RG, RP, LN, KH, JMR, GC, LM, JL, DP, LS, SH, CJ and CG all declare no support from any organisation for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Figures

Comment in
-
Validation of the 4C prediction models to inform care for patients with COVID-19: final steps towards clinical application.Thorax. 2022 Jun;77(6):536. doi: 10.1136/thoraxjnl-2021-218313. Epub 2022 Feb 2. Thorax. 2022. PMID: 35110368 No abstract available.
References
-
- NHS . C0860-clinical-commissioning-policy-remdesivir-for-people-hospitalised-with-covid-19-v2-.pdf. Available: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/... [Accessed 6 Apr 2021].
-
- NHS England . NHS England guidlines for Remdesivir therapy in COVID-19 patients. Available: https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2020/...
Publication types
MeSH terms
Associated data
Grants and funding
- MR/S032304/1/MRC_/Medical Research Council/United Kingdom
- 200 907/DH_/Department of Health/United Kingdom
- MC_PC_19026/MRC_/Medical Research Council/United Kingdom
- 205228/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_19059/MRC_/Medical Research Council/United Kingdom
- 200 927/DH_/Department of Health/United Kingdom
- 215091/Z/18/Z/WT_/Wellcome Trust/United Kingdom
- CO-CIN-01/DH_/Department of Health/United Kingdom
- IS-BRC-1215-20013/DH_/Department of Health/United Kingdom
- MC_PC_19025/MRC_/Medical Research Council/United Kingdom
- MC_PC_15001/MRC_/Medical Research Council/United Kingdom
- 201 385/DH_/Department of Health/United Kingdom
- MR/V001329/1/MRC_/Medical Research Council/United Kingdom
- 207511/Z/17/Z/WT_/Wellcome Trust/United Kingdom
- MC_UU_12014/9/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
