Physiology and Clinical Relevance of Enlarged Perivascular Spaces in the Aging Brain
- PMID: 34810243
- PMCID: PMC8792814
- DOI: 10.1212/WNL.0000000000013077
Physiology and Clinical Relevance of Enlarged Perivascular Spaces in the Aging Brain
Abstract
Perivascular spaces (PVS) are fluid-filled compartments that are part of the cerebral blood vessel wall and represent the conduit for fluid transport in and out of the brain. PVS are considered pathologic when sufficiently enlarged to be visible on MRI. Recent studies have demonstrated that enlarged PVS (ePVS) may have clinical consequences related to cognition. Emerging literature points to arterial stiffening and abnormal protein aggregation in vessel walls as 2 possible mechanisms that drive ePVS formation. We describe the clinical consequences, anatomy, fluid dynamics, physiology, risk factors, and in vivo quantification methods of ePVS. Given competing views of PVS physiology, we detail the 2 most prominent theoretical views and review ePVS associations with other common small vessel disease markers. Because ePVS are a marker of small vessel disease and ePVS burden is higher in Alzheimer disease, a comprehensive understanding about ePVS is essential in developing prevention and treatment strategies.
© 2021 American Academy of Neurology.
Figures
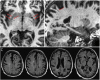
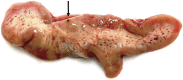

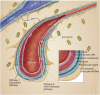

Comment in
-
The Perivascular Space Race: Understanding Their Role in Brain Clearance.Neurology. 2022 Jan 18;98(3):95-96. doi: 10.1212/WNL.0000000000013105. Epub 2021 Nov 22. Neurology. 2022. PMID: 34810242 No abstract available.
References
-
- Kida S, Steart PV, Zhang ET, Weller RO. Perivascular cells act as scavengers in the cerebral perivascular spaces and remain distinct from pericytes, microglia and macrophages. Acta Neuropathol. 1993;85(6):646-652. - PubMed
-
- Carare RO, Bernardes-Silva M, Newman TA, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34(2):131-144. - PubMed
-
- Kwee RM, Kwee TC. Tumefactive Virchow-Robin spaces. Eur J Radiol. 2019;111:21-33. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
