Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients With MS Using Different Disease-Modifying Therapies
- PMID: 34810244
- PMCID: PMC8826460
- DOI: 10.1212/WNL.0000000000013108
Humoral- and T-Cell-Specific Immune Responses to SARS-CoV-2 mRNA Vaccination in Patients With MS Using Different Disease-Modifying Therapies
Abstract
Background and objectives: To evaluate the immune-specific response after full severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination of patients with multiple sclerosis (MS) treated with different disease-modifying drugs by the detection of both serologic and T-cell responses.
Methods: Healthcare workers (HCWs) and patients with MS, having completed the 2-dose schedule of an mRNA-based vaccine against SARS-CoV-2 in the past 2-4 weeks, were enrolled from 2 parallel prospective studies conducted in Rome, Italy, at the National Institute for Infectious diseases Spallanzani-IRCSS and San Camillo Forlanini Hospital. Serologic response was evaluated by quantifying the region-binding domain (RBD) and neutralizing antibodies. Cell-mediated response was analyzed by a whole-blood test quantifying interferon (IFN)-γ response to spike peptides. Cells responding to spike stimulation were identified by fluorescence-activated cell sorting analysis.
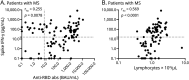
Results: We prospectively enrolled 186 vaccinated individuals: 78 HCWs and 108 patients with MS. Twenty-eight patients with MS were treated with IFN-β, 35 with fingolimod, 20 with cladribine, and 25 with ocrelizumab. A lower anti-RBD antibody response rate was found in patients treated with ocrelizumab (40%, p < 0.0001) and fingolimod (85.7%, p = 0.0023) compared to HCWs and patients treated with cladribine or IFN-β. Anti-RBD antibody median titer was lower in patients treated with ocrelizumab (p < 0.0001), fingolimod (p < 0.0001), and cladribine (p = 0.010) compared to HCWs and IFN-β-treated patients. Serum neutralizing activity was present in all the HCWs tested and in only a minority of the fingolimod-treated patients (16.6%). T-cell-specific response was detected in the majority of patients with MS (62%), albeit with significantly lower IFN-γ levels compared to HCWs. The lowest frequency of T-cell response was found in fingolimod-treated patients (14.3%). T-cell-specific response correlated with lymphocyte count and anti-RBD antibody titer (ρ = 0.554, p < 0.0001 and ρ = 0.255, p = 0.0078 respectively). IFN-γ T-cell response was mediated by both CD4+ and CD8+ T cells.
Discussion: mRNA vaccines induce both humoral and cell-mediated specific immune responses against spike peptides in all HCWs and in the majority of patients with MS. These results carry relevant implications for managing vaccinations, suggesting promoting vaccination in all treated patients with MS.
Classification of evidence: This study provides Class III data that SARS-CoV-2 mRNA vaccination induces both humoral and cell-mediated specific immune responses against viral spike proteins in a majority of patients with MS.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



Comment in
-
Response to SARS-CoV-2 vaccination in multiple sclerosis patients on disease modifying therapies.J Neurol. 2022 Apr;269(4):2259-2261. doi: 10.1007/s00415-022-11053-7. Epub 2022 Mar 14. J Neurol. 2022. PMID: 35286479 Free PMC article. No abstract available.
-
Comment: Humoral and T-cell Immunities to SARS-CoV-2 Vaccines: Safety, Efficacy, and Challenges in Autoimmune Neurology.Neurol Neuroimmunol Neuroinflamm. 2022 Jun 21;9(4):e200010. doi: 10.1212/NXI.0000000000200010. Print 2022 Jul. Neurol Neuroimmunol Neuroinflamm. 2022. PMID: 35728948 Free PMC article. No abstract available.
Comment on
-
Reading the "T" Leaves of COVID-19 Vaccine Responses in Multiple Sclerosis.Neurology. 2022 Feb 1;98(5):177-178. doi: 10.1212/WNL.0000000000013166. Epub 2021 Dec 8. Neurology. 2022. PMID: 34880090 No abstract available.
References
-
- McGinley MP, Goldschmidt CH, Rae-Grant AD. Diagnosis and treatment of multiple sclerosis: a review. JAMA. 2021;325:765-779. - PubMed
-
- Winkelmann A, Loebermann M, Reisinger EC, Hartung HP, Zettl UK. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol. 2016;12:217-233. - PubMed
-
- Pirttisalo AL, Sipilä JOT, Viitala M, Soilu-Hänninen M. Trends and characteristics of infection-related hospital admissions in multiple sclerosis patients in southwest Finland in 2009-2018. Mult Scler Relat Disord. 2020;44:102328. - PubMed
-
- Braun J, Loyal L, Frentsch M, et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270-274. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
