Rational regulation of water-seeking effort in rodents
- PMID: 34810265
- PMCID: PMC8640740
- DOI: 10.1073/pnas.2111742118
Rational regulation of water-seeking effort in rodents
Abstract
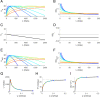
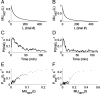
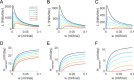
In the laboratory, animals' motivation to work tends to be positively correlated with reward magnitude. But in nature, rewards earned by work are essential to survival (e.g., working to find water), and the payoff of that work can vary on long timescales (e.g., seasonally). Under these constraints, the strategy of working less when rewards are small could be fatal. We found that instead, rats in a closed economy did more work for water rewards when the rewards were stably smaller, a phenomenon also observed in human labor supply curves. Like human consumers, rats showed elasticity of demand, consuming far more water per day when its price in effort was lower. The neural mechanisms underlying such "rational" market behaviors remain largely unexplored. We propose a dynamic utility maximization model that can account for the dependence of rat labor supply (trials/day) on the wage rate (milliliter/trial) and also predict the temporal dynamics of when rats work. Based on data from mice, we hypothesize that glutamatergic neurons in the subfornical organ in lamina terminalis continuously compute the instantaneous marginal utility of voluntary work for water reward and causally determine the amount and timing of work.
Keywords: circumventricular organs; elasticity of demand; neuroeconomics; thirst; wage–labor law.
Conflict of interest statement
The author declares no competing interest.
Figures




Similar articles
-
Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ.Nat Neurosci. 2017 Feb;20(2):230-241. doi: 10.1038/nn.4463. Epub 2016 Dec 19. Nat Neurosci. 2017. PMID: 27991901
-
Thirst driving and suppressing signals encoded by distinct neural populations in the brain.Nature. 2015 Apr 16;520(7547):349-52. doi: 10.1038/nature14108. Epub 2015 Jan 26. Nature. 2015. PMID: 25624099 Free PMC article.
-
The Forebrain Thirst Circuit Drives Drinking through Negative Reinforcement.Neuron. 2017 Dec 20;96(6):1272-1281.e4. doi: 10.1016/j.neuron.2017.11.041. Neuron. 2017. PMID: 29268095 Free PMC article.
-
From sensory circumventricular organs to cerebral cortex: Neural pathways controlling thirst and hunger.J Neuroendocrinol. 2019 Mar;31(3):e12689. doi: 10.1111/jne.12689. Epub 2019 Mar 14. J Neuroendocrinol. 2019. PMID: 30672620 Review.
-
Angiotensin and the lamina terminalis: illustrations of a complex unity.Clin Exp Hypertens A. 1988;10 Suppl 1:79-105. doi: 10.3109/10641968809075965. Clin Exp Hypertens A. 1988. PMID: 3072129 Review.
Cited by
-
Rats pursue food and leisure following the same rational principles.bioRxiv [Preprint]. 2025 Jul 31:2024.12.08.627420. doi: 10.1101/2024.12.08.627420. bioRxiv. 2025. PMID: 40766350 Free PMC article. Preprint.
-
Strategic stabilization of arousal boosts sustained attention.Curr Biol. 2024 Sep 23;34(18):4114-4128.e6. doi: 10.1016/j.cub.2024.07.070. Epub 2024 Aug 15. Curr Biol. 2024. PMID: 39151432
References
-
- Glimcher P., Fehr E., Neuroeconomics: Decision Making and the Brain (Academic Press, London, UK, 2014).
-
- Bhatti M., Jang H., Kralik J. D., Jeong J., Rats exhibit reference-dependent choice behavior. Behav. Brain Res. 267, 26–32 (2014). - PubMed

