The evolutionary conserved TLDc domain defines a new class of (H+)V-ATPase interacting proteins
- PMID: 34811399
- PMCID: PMC8608904
- DOI: 10.1038/s41598-021-01809-y
The evolutionary conserved TLDc domain defines a new class of (H+)V-ATPase interacting proteins
Erratum in
-
Author Correction: The evolutionary conserved TLDc domain defines a new class of (H+)V-ATPase interacting proteins.Sci Rep. 2021 Nov 30;11(1):23481. doi: 10.1038/s41598-021-02955-z. Sci Rep. 2021. PMID: 34848813 Free PMC article. No abstract available.
Abstract
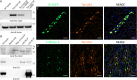
We recently found that nuclear receptor coactivator 7 (Ncoa7) and Oxr1 interact with the proton-pumping V-ATPase. Ncoa7 and Oxr1 belong to a group of proteins playing a role in the oxidative stress response, that contain the conserved "TLDc" domain. Here we asked if the three other proteins in this family, i.e., Tbc1d24, Tldc1 and Tldc2 also interact with the V-ATPase and if the TLDc domains are involved in all these interactions. By co-immunoprecipitation, endogenous kidney Tbc1d24 (and Ncoa7 and Oxr1) and overexpressed Tldc1 and Tldc2, all interacted with the V-ATPase. In addition, purified TLDc domains of Ncoa7, Oxr1 and Tldc2 (but not Tbc1d24 or Tldc1) interacted with V-ATPase in GST pull-downs. At the amino acid level, point mutations G815A, G845A and G896A in conserved regions of the Ncoa7 TLDc domain abolished interaction with the V-ATPase, and S817A, L926A and E938A mutations resulted in decreased interaction. Furthermore, poly-E motifs upstream of the TLDc domain in Ncoa7 and Tldc2 show a (nonsignificant) trend towards enhancing the interaction with V-ATPase. Our principal finding is that all five members of the TLDc family of proteins interact with the V-ATPase. We conclude that the TLDc motif defines a new class of V-ATPase interacting regulatory proteins.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

