Preparations from selected cucurbit vegetables as antiplatelet agents
- PMID: 34811441
- PMCID: PMC8608840
- DOI: 10.1038/s41598-021-02235-w
Preparations from selected cucurbit vegetables as antiplatelet agents
Abstract
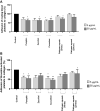
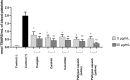
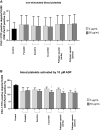
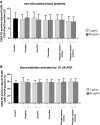
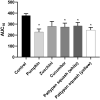
Increased blood platelet activation plays an important role in cardiovascular diseases (CVDs). Recent experiments indicate that certain fruits and vegetables, including onion, garlic, and beetroot, have anti-platelet potential and therefore may reduce the likelihood of CVDs. While vegetables from the Cucuritaceae family are known to exerting beneficial antioxidant and anti-inflammatory effects, their effects on blood platelet activation are poorly understood. Therefore, the aim of the present study was to determine the effect on platelet adhesion of preparations from selected cucurbits: pumpkin (Cucurbita pepo; fruit without seeds), zucchini (Cucurbita pepo convar. giromontina; fruit with seeds), cucumber (Cucumis sativus; fruit with seeds), white pattypan squash (Cucurbita pepo var. patisoniana; fruit without seeds) and yellow pattypan squash (Cucurbita pepo var. patisoniana, fruit without seeds). It also evaluates the activity of these preparations on enzymatic lipid peroxidation in thrombin-activated washed blood platelets by TBARS assay. The study also determines the anti-platelet properties of these five cucurbit preparations in whole blood by flow cytometry and with the total thrombus-formation analysis system (T-TAS) and evaluates the cytotoxicity of the tested preparations against platelets based on LDH activity. The results indicate that the yellow Cucurbita pepo var. patisoniana preparation demonstrated stronger anti-platelet properties than the other tested preparations, reducing the adhesion of thrombin-activated platelets to collagen/fibrinogen, and inhibiting arachidonic acid metabolism and GPIIb/IIIa expression on 10 µM ADP-activated platelets. None of the preparations was found to cause platelet lysis. Our findings provide new information on the anti-platelet activity of the tested cucurbit preparations and their potential for treating CVDs associated with platelet hyperactivity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

