MUC1 ectodomain is a flagellin-targeting decoy receptor and biomarker operative during Pseudomonas aeruginosa lung infection
- PMID: 34811449
- PMCID: PMC8608881
- DOI: 10.1038/s41598-021-02242-x
MUC1 ectodomain is a flagellin-targeting decoy receptor and biomarker operative during Pseudomonas aeruginosa lung infection
Abstract
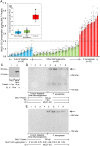
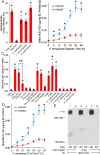
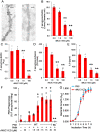
We previously reported that flagellin-expressing Pseudomonas aeruginosa (Pa) provokes NEU1 sialidase-mediated MUC1 ectodomain (MUC1-ED) desialylation and MUC1-ED shedding from murine lungs in vivo. Here, we asked whether Pa in the lungs of patients with ventilator-associated pneumonia might also increase MUC1-ED shedding. The levels of MUC1-ED and Pa-expressed flagellin were dramatically elevated in bronchoalveolar lavage fluid (BALF) harvested from Pa-infected patients, and each flagellin level, in turn, predicted MUC1-ED shedding in the same patient. Desialylated MUC1-ED was only detected in BALF of Pa-infected patients. Clinical Pa strains increased MUC1-ED shedding from cultured human alveolar epithelia, and FlaA and FlaB flagellin-expressing strains provoked comparable levels of MUC1-ED shedding. A flagellin-deficient isogenic mutant generated dramatically reduced MUC1-ED shedding compared with the flagellin-expressing wild-type strain, and purified FlaA and FlaB recapitulated the effect of intact bacteria. Pa:MUC1-ED complexes were detected in the supernatants of alveolar epithelia exposed to wild-type Pa, but not to the flagellin-deficient Pa strain. Finally, human recombinant MUC1-ED dose-dependently disrupted multiple flagellin-driven processes, including Pa motility, Pa biofilm formation, and Pa adhesion to human alveolar epithelia, while enhancing human neutrophil-mediated Pa phagocytosis. Therefore, shed desialylated MUC1-ED functions as a novel flagellin-targeting, Pa-responsive decoy receptor that participates in the host response to Pa at the airway epithelial surface.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 Clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin. Infect. Dis. 2016;63:575–582. doi: 10.1093/cid/ciw504. - DOI - PMC - PubMed
-
- Edwards JR, Peterson KD, Andrus ML, Tolson JS, Goulding JS, Dudeck MA, Mincey RB, Pollock DA, Horan TC. NHSN facilities, national healthcare safety network (NHSN) report, data summary for 2006, issued June 2007. Am. J. Infect. Control. 2007;35:290–301. doi: 10.1016/j.ajic.2007.04.001. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

