HIV-1 Vif suppresses antiviral immunity by targeting STING
- PMID: 34811497
- PMCID: PMC8752805
- DOI: 10.1038/s41423-021-00802-9
HIV-1 Vif suppresses antiviral immunity by targeting STING
Abstract
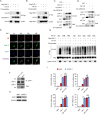
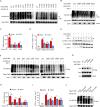
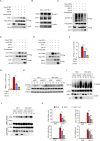
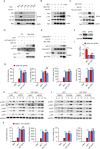
HIV-1 infection-induced cGAS-STING-TBK1-IRF3 signaling activates innate immunity to produce type I interferon (IFN). The HIV-1 nonstructural protein viral infectivity factor (Vif) is essential in HIV-1 replication, as it degrades the host restriction factor APOBEC3G. However, whether and how it regulates the host immune response remains to be determined. In this study, we found that Vif inhibited the production of type I IFN to promote immune evasion. HIV-1 infection induced the activation of the host tyrosine kinase FRK, which subsequently phosphorylated the immunoreceptor tyrosine-based inhibitory motif (ITIM) of Vif and enhanced the interaction between Vif and the cellular tyrosine phosphatase SHP-1 to inhibit type I IFN. Mechanistically, the association of Vif with SHP-1 facilitated SHP-1 recruitment to STING and inhibited the K63-linked ubiquitination of STING at Lys337 by dephosphorylating STING at Tyr162. However, the FRK inhibitor D-65495 counteracted the phosphorylation of Vif to block the immune evasion of HIV-1 and antagonize infection. These findings reveal a previously unknown mechanism through which HIV-1 evades antiviral immunity via the ITIM-containing protein to inhibit the posttranslational modification of STING. These results provide a molecular basis for the development of new therapeutic strategies to treat HIV-1 infection.
Keywords: FRK; Immune evasion; Vif; cGAS–STING.
© 2021. The Author(s), under exclusive licence to CSI and USTC.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Gringhuis SI, den Dunnen J, Litjens M, van der Vlist M, Geijtenbeek TB. Carbohydrate-specific signaling through the DC-SIGN signalosome tailors immunity to Mycobacterium tuberculosis, HIV-1 and Helicobacter pylori. Nat Immunol. 2009;10:1081–8. - PubMed
-
- Gringhuis SI, van der Vlist M, van den Berg LM, den Dunnen J, Litjens M, Geijtenbeek TB. HIV-1 exploits innate signaling by TLR8 and DC-SIGN for productive infection of dendritic cells. Nat Immunol. 2010;11:419–26. - PubMed
-
- Jakobsen MR, Olagnier D, Hiscott J. Innate immune sensing of HIV-1 infection. Curr Opin HIV AIDS. 2015;10:96–102. - PubMed
-
- Heil F, Hemmi H, Hochrein H, Ampenberger F, Kirschning C, Akira S, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

