Mitochondrial aspartate regulates TNF biogenesis and autoimmune tissue inflammation
- PMID: 34811544
- PMCID: PMC8756813
- DOI: 10.1038/s41590-021-01065-2
Mitochondrial aspartate regulates TNF biogenesis and autoimmune tissue inflammation
Abstract
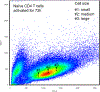
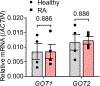
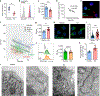
Misdirected immunity gives rise to the autoimmune tissue inflammation of rheumatoid arthritis, in which excess production of the cytokine tumor necrosis factor (TNF) is a central pathogenic event. Mechanisms underlying the breakdown of self-tolerance are unclear, but T cells in the arthritic joint have a distinctive metabolic signature of ATPlo acetyl-CoAhi proinflammatory effector cells. Here we show that a deficiency in the production of mitochondrial aspartate is an important abnormality in these autoimmune T cells. Shortage of mitochondrial aspartate disrupted the regeneration of the metabolic cofactor nicotinamide adenine dinucleotide, causing ADP deribosylation of the endoplasmic reticulum (ER) sensor GRP78/BiP. As a result, ribosome-rich ER membranes expanded, promoting co-translational translocation and enhanced biogenesis of transmembrane TNF. ERrich T cells were the predominant TNF producers in the arthritic joint. Transfer of intact mitochondria into T cells, as well as supplementation of exogenous aspartate, rescued the mitochondria-instructed expansion of ER membranes and suppressed TNF release and rheumatoid tissue inflammation.
© 2021. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Declaration of Interests
The authors have declared that no conflict of interest exists.
Figures




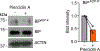








Comment in
-
Shortage of aspartate in mitochondria fuels arthritis.Nat Immunol. 2021 Dec;22(12):1474-1476. doi: 10.1038/s41590-021-01069-y. Nat Immunol. 2021. PMID: 34811543 Free PMC article.
-
Mitochondrial signals regulate ER size and TNF production in rheumatoid arthritis.Nat Immunol. 2021 Dec;22(12):1477-1478. doi: 10.1038/s41590-021-01070-5. Nat Immunol. 2021. PMID: 34837082 No abstract available.
-
Mitochondria Transfer to CD4+ T Cells May Alleviate Rheumatoid Arthritis by Suppressing Pro-Inflammatory Cytokine Production.Immunometabolism. 2022;4(2):e220009. doi: 10.20900/immunometab20220009. Epub 2022 Mar 18. Immunometabolism. 2022. PMID: 35444834 Free PMC article.
References
References for Methods
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

